Actins from different species even as far apart as plants, yeasts, and mammals bear a striking similarity in amino acid sequence. For example, actin from Acanthamoeba differs from mammalian in only 15 out of 375 amino acids, while a comparison of skeletal muscle actin from various species of warm-blooded vertebrates fails to reveal any amino acid change at all. Few other proteins exhibit this striking evolutionary consistency. One cited explanation for this is the very large number of protein-protein interactions actin has to fulfill, that it is difficult for such a protein to change its amino acid sequence without adversely affecting one or more of its multiple tasks in the cell. In the digital video presented above, embryonic rat thoracic aorta fibroblast cells (A7r5 line) are expressing a fusion of Dronpa fluorescent protein to human beta-actin.
Video 1 - Run Time: 17 Seconds
Video 2 - Run Time: 08 Seconds
Video 3 - Run Time: 03 Seconds
Video 4 - Run Time: 07 Seconds
Video 5 - Run Time: 04 Seconds
Video 6 - Run Time: 07 Seconds
Video 7 - Run Time: 08 Seconds
Video 8 - Run Time: 07 Seconds
Video 9 - Run Time: 11 Seconds
Video 10 - Run Time: 16 Seconds
Video 11 - Run Time: 06 Seconds
Six distinct actin isotypes have been identified in mammalian cells. Each is encoded by a separated gene and is expressed in a developmentally regulated and tissue-specific manner. alpha and beta cytoplasmic actins are expressed in a wide variety of cells, while expression of alpha skeletal, alpha cardiac, alpha vascular, and gamma enteric actins are more restricted to specialized muscle cell type. Smooth muscle alpha actin is of further interest because it is one of a few genes whose expression is relatively restricted to vascular smooth muscle cells. Expression of smooth muscle alpha actin is regulated by hormones, cell proliferation, and is altered by pathological conditions including oncogenic transformation (the properties by which cells become cancerous) and atherosclerosis (a disease affecting arterial blood vessels). In the digital video presented above, embryonic rat thoracic aorta fibroblast cells (A7r5 line) are expressing a fusion of Dronpa fluorescent protein to human beta-actin.
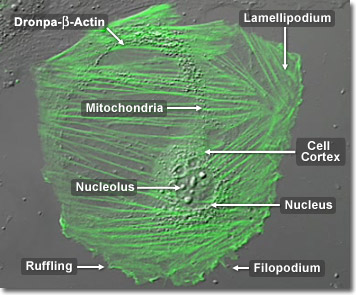
Ubiquitous and highly versatile, actin is involved in the movement of every known eukaryotic cell. From cytokinesis and phagocytosis (the digestion by cells of solid substances) to cytoplasmic streaming and cell crawling, all depend on structures built from actin. Actin molecules group into static bundles, contractile bundles, two-dimensional networks, and three-dimensional gels. Each grouping has a characteristic structure, specified by a collection of different actin-binding proteins. In the digital video presented above, embryonic rat thoracic aorta fibroblast cells (A7r5 line) are expressing a fusion of Dronpa fluorescent protein to human beta-actin.