Actin filaments are composed of polymerized globular actin subunits and are found throughout the cytoplasm of the cell, but concentrated just underneath the surface of the cell plasma membrane in a region known as the cortex. From the cortex, actin filaments spread out in all directions to provide structural support. Whereas microtubules direct intracellular traffic, actin filaments are responsible for the cell's overall mobility in extra-cellular media. An individual actin filament is wound into a 2-stranded helix, which may be several micrometers in length with a diameter of 5 to 9 nanometers. The filaments are quite flexible structures that can be organized into bundles, networks, or three-dimensional gels. In the digital video presented above, normal Gray fox lung fibroblast cells (FoLu line) expressing a chimera of monomeric Kusabira Orange fluorescent protein fused to human beta-actin are observed in a time-lapse sequence recorded under a combination of fluorescence and differential interference contrast (DIC) illumination.
Video 1 - Run Time: 59 Seconds
Video 2 - Run Time: 51 Seconds
Video 3 - Run Time: 57 Seconds
Video 4 - Run Time: 56 Seconds
Video 5 - Run Time: 60 Seconds
Video 6 - Run Time: 60 Seconds
Video 7 - Run Time: 55 Seconds
Video 8 - Run Time: 51 Seconds
Video 9 - Run Time: 50 Seconds
Video 10 - Run Time: 12 Seconds
Video 11 - Run Time: 46 Seconds
Video 12 - Run Time: 61 Seconds
Video 13 - Run Time: 51 Seconds
Video 14 - Run Time: 55 Seconds
Video 15 - Run Time: 54 Seconds
Video 16 - Run Time: 51 Seconds
Video 17 - Run Time: 56 Seconds
Actin is a highly conserved protein that has changed very little over the course of time. This observation is verified by the fact that the amino acid sequence coding for actin in humans is similar to that found in plants and yeasts. Globular actin monomers bond readily to each other to form two parallel polar filaments arranged into a helical array. The consequence of this structural polarity is that the two ends of the filaments polymerize and depolymerize at different rates depending on which portion of the actin monomer's molecular surface is exposed. Assembling the central section first, the actin filament grows linearly from both ends of a central nucleating oligomer. The two polar termini of an actin filament are referred to as the "barbed" and "pointed" ends, with the barbed end usually polymerizing faster than the pointed end. In the digital video presented above, normal Gray fox lung fibroblast cells (FoLu line) expressing a chimera of monomeric Kusabira Orange fluorescent protein fused to human beta-actin are observed in a time-lapse sequence recorded under a combination of fluorescence and differential interference contrast (DIC) illumination.
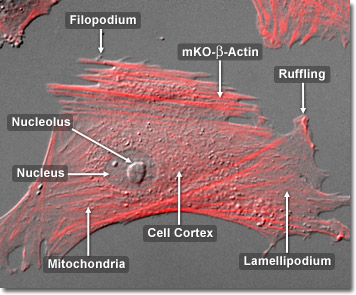
In the cells of living organisms, actin filaments are usually organized into large structures by various accessory proteins. The exact structural form that a group of actin microfilaments assumes depends on their primary function and the particular proteins that bind them together. For example, in the core of surface protrusions called microspikes, microfilaments are organized into tight parallel bundles by the bundling protein, fimbrin. Bundles of the filaments are less tightly packed together, however, when they are bound by alpha-actinin or are associated with fibroblast stress fibers. Notably, the microfilament connections created by some cross-linking proteins result in a web-like network, or gel, form rather than filament bundles. In the digital video presented above, normal Gray fox lung fibroblast cells (FoLu line) expressing a chimera of monomeric Kusabira Orange fluorescent protein fused to human beta-actin are observed in a time-lapse sequence recorded under a combination of fluorescence and differential interference contrast (DIC) illumination.