Tackling diabetes with confocal microscopy
Abstract
Research efforts addressing the global challenge of Diabetes Mellitus are very much dependent on applying advanced microscopy technologies, but such equipment can often be inaccessible for many researchers. Addressing the everyday demands of life science research groups, a new category of compact all-in-one confocal microscope has emerged to enable students and professors alike to achieve high quality results.
The FluoView FV10i from Olympus is proving to be a popular toolin the research group of Professor Simone Baltrusch from the Medical Faculty, University of Rostock. Meeting with Olympus Application Specialist Dr. Helge Schmidt, she discusses how accessible options for confocal microscopy have boosted her group’s work – advancing understanding of the role played by the mitochondrial network and facilitating the development of techniques for diagnosing neuropathy.
If current trends continue, it is predicted that by the year 2035, one adult in ten will suffer from Diabetes Mellitus, a disease that is becoming an ever more pressing medical challenge.1

The Team
Professor Dr Simone Baltrusch (back row, middle) and part of her research group (from back left to front right: Rica Waterstradt, Dr. Julia Schultz, Annett Kott, Janine Leckelt, Jan Niemann) in the laboratory with the Olympus FluoView FV10i.
One of the key factors underlying this metabolic disorder is the impaired function of the insulin-producing β-cells of the pancreas, with a subsequent drop in insulin secretion leading to abnormally elevated blood sugar levels and resulting in a variety of physiological complications. While type 1 diabetes stems from the autoimmune destruction of β-cells, type 2 diabetes is characterized by both β-cell failure and insulin resistance in tissues around the body. Understanding the mechanisms and implications of this complex disorder is highly important, and Professor Simone Baltrusch from the Medical Faculty, University of Rostock, has found confocal laser scanning microscopy to be an ideal tool for this.
Compared to standard widefield microscopy, confocal laser scanning microscopy delivers a sharp image derived only from a defined focal plane. As confocal technologies have progressed, it has been an interesting development that, while complex modular systems such as those built on multiphoton technology enable the most cutting edge in vivo investigations, a new category of compact all-in-one system has also emerged to address the everyday demands of the busy life science
research laboratory. Allowing every member of Professor Simone Baltrusch’s group to benefit from confocal microscopy, one such system is the FluoView FV10i from Olympus. “We chose the FV10i because not everyone is familiar with microscopy, and we needed a system that is easy to work with, and this has proved to be the case,” she comments, adding: “We have a lot of students working on their medical theses. They quickly become independent analyzing
their samples with this machine,which never happened in the past.” This is also important where the two post-doctoral researchers in the group look after many students. Between teaching and research, time can be limited for introducing new students, so a user-friendly system means the supervisor is in the background if something happens, but the students can achieve good results by themselves.
Prof. Baltrusch’s group relies on this system to study many aspects of diabetes, not only at the molecular level2 but also studying wider aspects of the disease in relation to the affected organelles and cells. She discusses two interesting projects:Understanding the role of mitochondrial mutations underlying diabetes, and quantifying the hyperglycaemia-induced damage to neuronal networks within the eye.
Updating the Textbooks: the Diabetic Mitochondrial Network
As knowledge of biological systems progresses, it is becoming increasingly clear that what were once considered static components are instead highly interconnected networks. This is even true of the mitochondria, with Prof. Baltrusch explaining: “Textbook diagrams of mitochondria within the cell often show separated organelles with maybe 10-20 per cell, but this is not actually the case. This is a dynamic network undergoing a continuous process of fusion and fission - it’s changing all the time.”
Interestingly, a correlation has been observed between type 2 diabetes and an increase in mitochondrial mutations. Carrying mitochondrial mutations and a wild type nucleus, conplastic mouse models are ideal for understanding this relationship.3
“We want to know what happens when such mutations accumulate over time,” says Prof. Baltrusch. “Does the mitochondrial network structure or function change? Studying mice carrying such mutations, we can see what happens compared to the wild type strain.”
Developing Diabetes Disease Models
The focus on diabetes is also part of a larger research focus on ageing and diseases at the University of Rostock, with this collaboration including Professor Markus Tiedge’s research group*. Developing mouse models of diabetes is important in this context, as Prof. Baltrusch explains most current research into ageing uses worms: “This is much easier –with a lifetime of 30 days, if a new strain survives 33 days, you might conclude it’s living longer, but to transfer this to humans is difficult.” For studying mitochondrial mutations and neuronal damage in diabetes, mammalian models provide a more accurate representation of humans.

Researcher Janine Leckelt works with the group’s diabetic mouse models at the University of Rostock
Figure 1 shows a primary mouse hepatocyte cultured and visualized ex vivo using confocal microscopy. Following image processing, individual mitochondria could be differentiated and quantified to detect any that were abnormally elongated or fragmented. The quality of the acquired images has been central to this level of analysis, and the Olympus FV10i has proved to be popular within the institute. “When we first started with the FV10i on this project, we showed some of our results at internal workshops here at the university. Our audiences were really impressed, and neighboring groups who already have confocal microscopes, a lot of them have approached us, asking if they can use this machine. If you check our booking plan, it is totally full. Even running overnight it’s completely booked.”
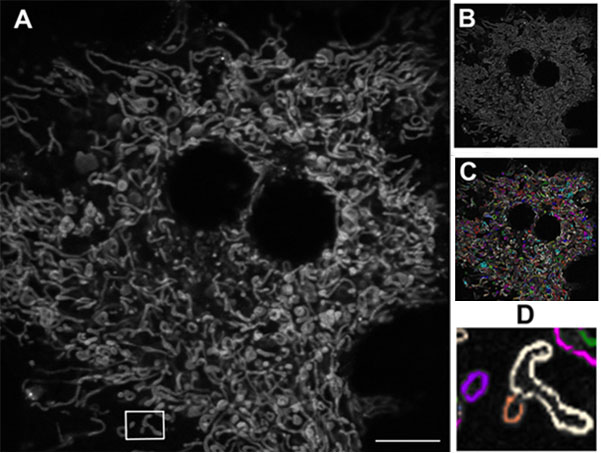
Figure 1: Mitochondrial network in a living mouse hepatocyte.
A) Original image. B) Following edge-finding processing (Image J software, NIH). C) Individual mitochondria (Autoquant software, Media Cybernetics) D) Elongated mitochondria (white) and fragmented mitochondria (purple and orange). Using the Olympus FV10i, 60x oil objective. Scale bar 10 μm.
The increased mitochondrial fragmentation observed within diabetes means it becomes problematic to get enough energy for cells, and has been implicated in insulin resistance of skeletal muscle.4 On the other hand, very elongated mitochondria are also problematic. Interestingly, studying images from different networks, Prof. Baltrusch’s group has found this is very much dependent on cell type and physiological conditions. “The mitochondrial network adapts to what is needed, how much energy is available and where this comes from – from fat or carbohydrate. Each affects the network, and we’ve really learnt a lot.”
Towards Non-Invasive Detection of Diabetic Neuropathy
Whilst understanding the underlying mechanisms of diabetes is vital for developing new treatments and advancing our knowledge of biological systems, it is equally valuable to look at the impact diabetes has on quality of life.
A second project utilizing the Olympus FV10i works towards the development of a new technique for early detection of diabetic neuropathy, or neuronal damage. When hyperglycaemia (an elevated blood sugar level) persists over five years or longer, physiological damage can be quite expansive – and neuropathy is the most common long-term complication of diabetes. It arises when glycation of blood proteins gives rise to what are known as advanced glycation end products (AGEs), which bind receptors on the surface of neurons and trigger damaging processes such as apoptosis.
Early diagnosis is therefore crucial for disease management, and yet current techniques are non-quantifiable, depending on the patient’s verbal response to a sensory trigger applied to the foot. A more quantitative technique is needed, and while analyzing the neuronal network from a skin biopsy can identify any fibers lost, this is painful and wound healing is also a problem for diabetics. A collaborative project with Professor Oliver Stachs’s group from the Department of Ophthalmology** instead looks at analyzing neuronal networks in the cornea of the eye. Using a specially designed corneal confocal microscopy system, the team is able to visualize the subbasal nerve plexus of the cornea in humans.5
This non-invasive approach lends itself to the detection of diabetic neuropathy, and through understanding more about the structure of corneal nerves, this project works towards developing its use as a quantitative technique within the clinic.
Working with diabetic mouse models in Prof. Baltrusch’s group, the Olympus FV10i is applied to quantify both nerve density and length within isolated corneas ex vivo, providing insights into diabetic neuropathy and guiding the most effective use of in vivo corneal confocal microscopy.
The Diabetic Mouse Model
With a focus on a type of diabetes not triggered by autoimmunity, s-cells of the pancreatic islet cells are specifically destroyed using the compound streptozotocin. Samples are investigated using antibody staining for insulin, and visualized using confocal microscopy, with Figure 2A showing an intact pancreatic islet within a fixed pancreatic section. The shape is comparable to a living pancreatic islet isolated by collagenase digestion of the mouse pancreas, as seen in Figure 2B.
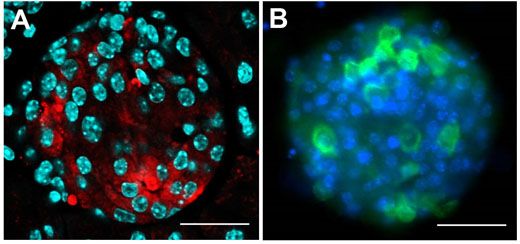
Figure 2: Investigating the pancreatic islet cells in mouse.
The insulin-producing β-cells were investigated within this pancreatic islet of Langerhans,and visualised with confocal microscopy. A) Pancreatic section staining for insulin (red), with nuclei in cyan. B) Pancreatic islet cells in culture, with insulin (green) and nuclei stained in blue. Using the Olympus FV10i with 60x oil objective; scale bar 50 μm.
Finding the Right Nerves
Using a transgenic mouse with nerve fibers labelled with yellow fluorescent protein (the thy1-YFP mouse), corneal nerves are visualized ex vivo using the Olympus FV10i. Figure 3 shows the larger stromal nerve and the very thin nerves of the subbasal nerve plexus. However, the cellular architecture of the cornea is complex.6 As can be seen in Figure 4, this nerve plexus is close to the Bowman’s layer, arises from the penetrating A delta fibers, and has subbasal orientation. Prof. Baltrusch comments: “The subbasal nerves are very sensitive to damage, and you see a loss of these in people and animals with diabetes.” Comparing old and new techniques, skin samples from the mice are also analyzed, and corneal nerves are found to be much more susceptible – owing to the presence of more receptors for AGEs.
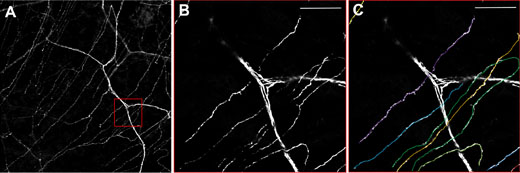
Figure 3: Neurones in the cornea of a thy1-YFP mouse.
A) Overview showing the thicker stromal nerves and the finer nerves of the subbasal plexus. B)
Zooming in on the red box and C) Quantification of single subbasal nerves. Using the FV10i, 60x oil objective. Scale bar 50 μm.
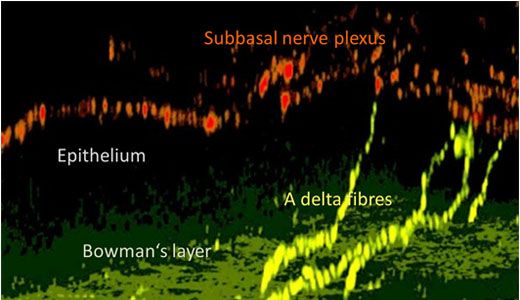
Figure 4: Nerve structure in the mouse cornea.
In this 3D reconstruction using confocal microscopy, A delta fibres (yellow) penetrate the Bowman’s layer. Thesvve fibres spread into the subbasal nerve plexus (red).
She goes on to explain: “It is crucial to find the right layer of the cornea and analyzed the correct nerves, but being so thin this can be difficult.” Confocal microscopy is ideally suited to this application, as it allows selection of the correct z-position and facilitates analysis with 3D imaging.
The Bigger Picture
One question in this study is how many pictures are required to get a good representation of corneal neurone health. In addition to nerve density, nerve fibre length is also an indicator of neuronal health, and this requires a large field of view.
Acquiring a larger field of view with the FV10i is achieved using the automatic image stitching function. “One of the main reasons why we chose the FV10i is for image quantification and high-throughput analysis,” Prof. Baltrusch comments. “With image stitching on the FV10i, we get a very nice overview.”
Figure 5 shows an acquisition of 36 images with 25 z-slices generated overnight, while imaging one quarter of the cornea takes three days. Throughout these extended experiments, the machine runs autonomously, with the autofocus routine of the FV10i ensuring the scan field remains in focus between multiple images. This is not standard on confocal setups and, in particular, not for a compact all-in-one system. The operator would otherwise need to be working at the machine to focus each image, and also need experience to acquire a high quality stitched image. For quantifying nerve fiber density and the length of individual nerves, image stitching yields a much more insightful representation of the cornea. “Ideally we want to look at the whole cornea,” says Prof. Baltrusch. Although the FV10i is able to acquire the images, this data load requires specialized software for image analysis and quantification. While the whole cornea would amount to 160 GB, while even the stitched image shown in Figure 5 taken from less than a quarter of the cornea constitutes approximately 6 GB.
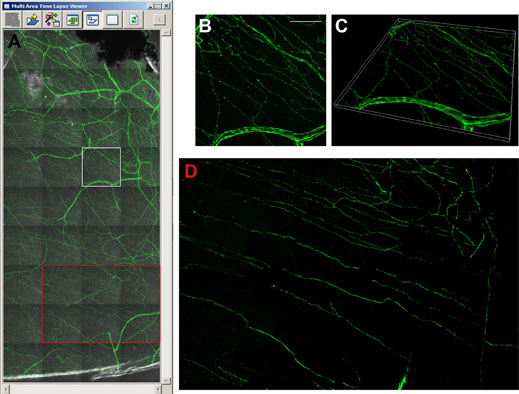
Figure 5: Visualising the thy1-YFP mouse cornea with image stitching.
A) Multi area time laps viewer shows 36 single images with 25 z-layers each. The image in the marked white square is shown as a 3D maximum projection (B), and as a 3D volume view (C). Image stitching facilitates analysis of subbasal corneal nerves (red square in A, and D). Using the FV10i, 60x oil objective. Scale bar 50 μm.
Future Investigations
In addition to facilitating the detection of neuropathy, these investigations have opened other avenues in research, as Prof.Baltrusch says: “We are interested in which areas are damaged first, and when mice are treated with insulin, what comes back first? What is really interesting, is that you can see [in Figure 5B] these dots in the nerves, and we know that these beaded nerve fibers branch, and thus are important for new nerves.” Combining accurate diabetic mouse models and both in vivo and ex vivo confocal microscopy tools is also the ideal platform for testing new compounds for treating diabetic neuropathy.
Summary
Diabetes Mellitus is a complex disease linked to a variety of causal factors and implications, and confocal microscopy with the Olympus FV10i has been central to the research of Professor Baltrusch and her group. Enabling detailed and high-throughput analysis, the FV10i combines powerful confocal microscopy in a user-friendly system, advancing understanding of the role played by the mitochondrial network structure and facilitating the development of techniques for diagnosing neuropathy.
“Even for those without microscopy experience, we can now analyze approximately 1,000 samples throughout all tissues to create a picture of diabetes in the whole organism.” Allowing students and professors alike to generate high-quality results is a key driver for advancing academic research and helping in the fight against diabetes.
Information
Professor Dr Simone Baltrusch is vice-director of the Institute for Medical Biochemistry and Molecular Biology, University of Rostock (Germany).
Collaborations
* The group of Professor Markus Tiedge, director of the Institute of Medical Biochemistry and Molecular Biology, in addition to collaborating institutes at the Medical Faculty, University of Rostock
** Professor Oliver Stachs, group leader of the Department of Ophthalmology at the Medical Faculty, University of Rostock
References
1. International Diabetes Federation (2013). IDF Diabetes Atlas Sixth edition.
www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf
2. Hofmeister-Brix A., et al. (2013) Identification of the ubiquitin-like domain of midnolin as a new glucokinase interaction partner. JBC 288(50):35824-39.
3. Yu X., et al. (2009). Dissecting the effects of mtDNA variations on complex traits using mouse conplastic strains.
Genome Res. 19(1):159-65.
4. Jheng H.F., et al. (2012). Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell Biol. 32(2):309-19.
5. Zhivov A, et al. (2013) Imaging and Quantification of Subbasal Nerve Plexus in Healthy Volunteers and Diabetic Patients with or without Retinopathy. PLoS ONE 8(1): e52157. doi:10.1371/journal.pone.0052157
6. Guthoff R.F, et al. (2005) Epithelial Innervation of Human Cornea. Cornea 24(5): 608-613
Produtos usados nesta aplicação
foi adicionado com sucesso aos seus favoritos
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.