Not Available in Your Country
Sorry, this page is not
available in your country.
Overview
 | Confocal Imaging of Rapid Cell DynamicsThe IXplore Spin microscope system’s advanced spinning disk unit contributes to a large field of view, fast 3D confocal image acquisition, and prolonged cell viability in time-lapse experiments. |
|---|
High-Speed Confocal ImagingThe Yokogawa CSU-W1 spinning disk unit helps you acquire high-speed confocal images with a larger field of view than traditional confocal microscopes. Olympus’ TruSight deconvolution algorithms can be applied to improve image resolution, contrast, and dynamic range for strikingly clear 3D images, even at greater observation depths. |
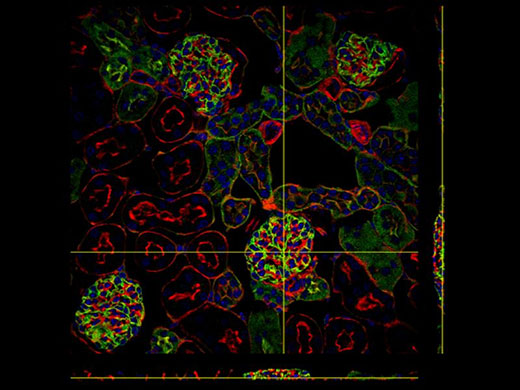
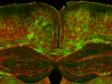
*Kidney Section Slide (Blue: DAPI, Green: WGA, Red: Phalloidin) |
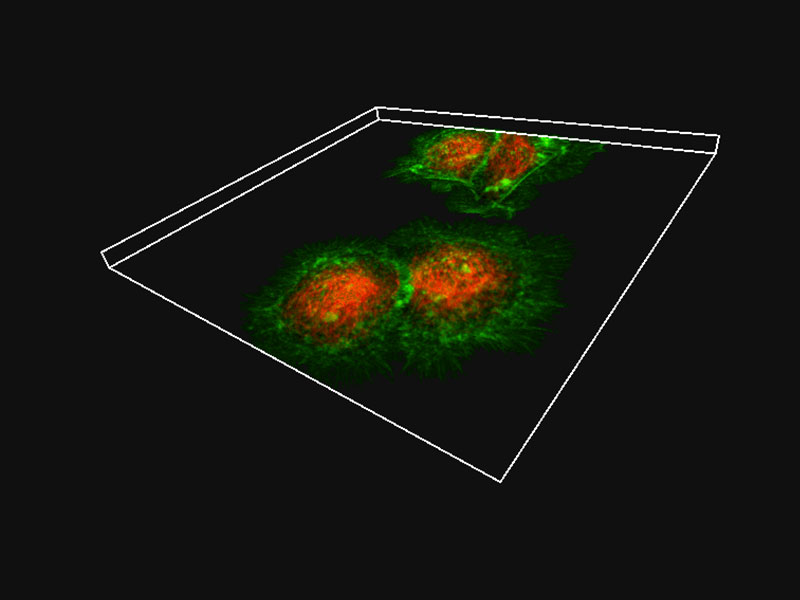 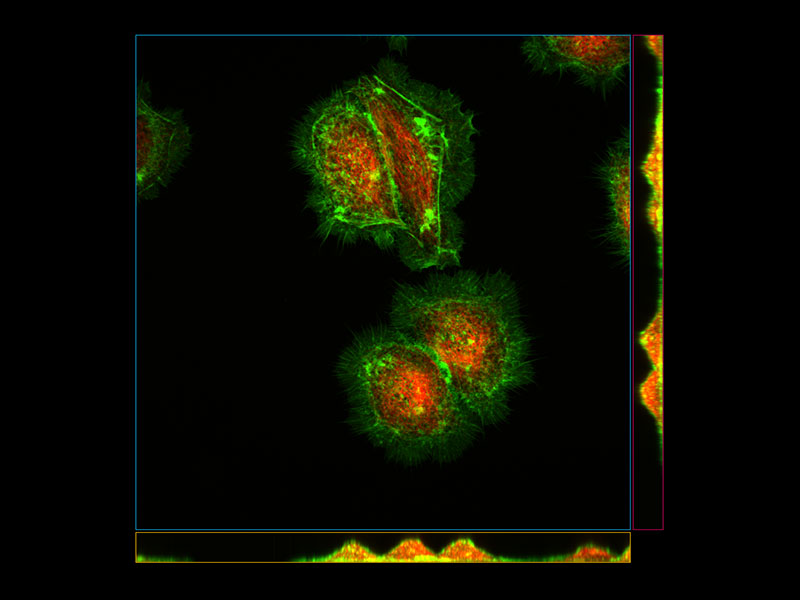 | Precise 3D ImagingThe pinhole geometry of the Yokogawa spinning disk produces excellent image contrast at greater depths for imaging into thicker samples. Additionally, the IXplore Spin system combines high numerical aperture silicone oil objectives with ergonomic spherical aberration correction collar adjustment to provide excellent light gathering and dimensional fidelity. These elements make the IXplore Spin system the right choice for researchers who need to image living cells at high resolution without sacrificing speed, accuracy, or image quality. *Image: HeLa cells (Green: Actin, Red: Tubulin)*1 |
|---|
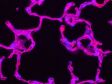
Upgrade to SpinSRThe IXplore microscope system is designed to meet your evolving research needs. The SpinSR super resolution module is available as an upgrade to any existing Olympus IXplore system and can be configured to fit your budget. *Image: Fluorescent staining of microtubules (red: Alexa 594) and actin (green: Alexa 488 phalloidin) in growth cone of NG108 cells. Image courtesy of: Dr.Kaoru Katoh , Biomedical Research Institute, National Institute of Advanced Industrial Sciences and Technology (AIST) |  Left: Confocal / Right: Super Resolution |
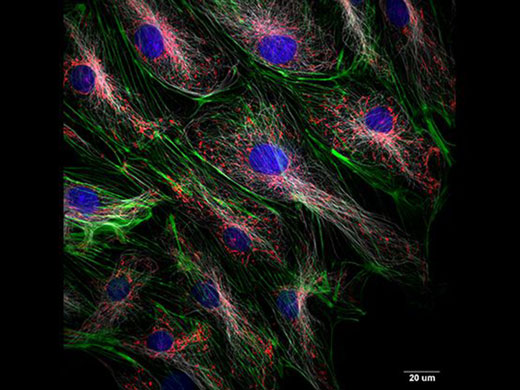 | Simultaneous Multicolor Confocal ImagingThe IXplore Spin laser combiner is scalable from two to six laser lines. A dual camera option is available to support simultaneous multichannel imaging when higher speed and a wider information bandwidth are required. Available excitation wavelengths include 405 nm, 445 nm, 488 nm, 514 nm, 561 nm, and 640 nm. *Image: Rat-kangaroo Epithelial Kidney cells (Blue: Nuclear, Green: Actin, Red: Mitochondria, White: Tubulin) |
|---|
High Contrast under Bright ConditionsDesigned for fluorescence observation, the Umbra light shield blocks out light pollution, even in bright rooms, enhancing fluorescence contrast and improving image clarity. |
|
ReferencesN. Elkhatib, et al. Tubular clathrin/AP-2 lattices pinch collagen fibers to support 3D cell migration. Science (June 16, 2017). R. H. Herbst, et al. Heterosis as a consequence of regulatory incompatibility. BMC Biology (May 11, 2017). N. Yanagisawa, et al. Capability of tip-growing plant cells to penetrate into extremely narrow gaps (May 3, 2017). H. Cohen-Dvashi, et al. The role of LAMP1 binding and pH sensing by the spike complex of Lassa virus. Journal of Virology (September 7, 2016). H. Ochiai, et al. Simultaneous live imaging of the transcription and nuclear position of specific genes. Nucleic Acids Research (June 19, 2016). B. Guirao, et al. Unified quantitative characterization of epithelial tissue development. eLIFE (December 12, 2015). I. Nemazanyy, et al. Class III PI3K regulates organismal glucose homeostasis by providing negative feedback on hepatic insulin signalling. Nature Communications (September 21, 2015). K. Gooh, et al. Live-cell imaging and optical manipulation of arabidopsis early embryogenesis. Developmental Cell (July 9, 2015). Y. Oda, et al. Rho of plant GTPase signaling regulates the behavior of arabidopsis kinesin-13A to establish secondary cell wall patterns. The Plant Cell (November 26, 2013). |
*1 Although it became one of the most important cell lines in medical research, it’s imperative that we recognize Henrietta Lacks’ contribution to science happened without her consent. This injustice, while leading to key discoveries in immunology, infectious disease, and cancer, also raised important conversations about privacy, ethics, and consent in medicine.
|
Need assistance? |
Specifications
| Microscope Frame | IX83P2ZF | |
|---|---|---|
| Observation Method > Confocal | ✓ | |
| Observation Method > Fluorescence (Blue/Green Excitation) | ✓ | |
| Observation Method > Fluorescence (Ultraviolet Excitation) | ✓ | |
| Observation Method > Differential Interference Contrast (DIC) | ✓ | |
| Observation Method > Phase Contrast | ✓ | |
| Observation Method > Brightfield | ✓ | |
| Revolving Nosepiece > Motorized (6 position) | ✓ | |
| Focus > Motorized |
| |
| Focus > Z Drift Compensator | ✓ | |
| Observation Tubes > Widefield (FN 22) > Tilting Binocular | ✓ | |
| Illuminator > Transmitted Köhler Illuminator > LED Lamp | ✓ | |
| Illuminator > Transmitted Köhler Illuminator > 100 W Halogen Lamp | ✓ | |
| Illuminator > Fluorescence Illuminator > 100 W Mercury Lamp | ✓ | |
| Illuminator > Fluorescence Illuminator > Light Guide Illumination | ✓ | |
| Fluorescence Mirror Turret > Motorized (8 position) | ✓ | |
| Stage > Motorized | Contact your local sales representative to hear about motorized stage options | |
| Stage > Mechanical > IX3-SVR Mechanical Stage with Right Handle |
| |
| Stage > Mechanical > IX3-SVL Mechanical Stage with Left Short Handle |
| |
| Condenser > Motorized > Universal Condenser | W.D. 27 mm, NA 0.55, motorized aperture and polarizer | |
| Condenser > Manual > Universal Condenser | NA 0.55/ W.D. 27 mm | |
| Condenser > Manual > Ultra-Long Working Distance Condenser | NA 0.3/ W.D. 73.3 mm | |
| Confocal Scanner | CSU-W1 | |
| Super Resolution Processing | - | |
| Accessories | Remote correction collar controller (IX3-RCC) | |
| Dimensions (W × D × H) | 323 (W) x 475 (D) x 706 (H) mm (IX83 microscope frame) | |
| Weight | Approx. 47kg (IX83P2ZF) |