Not Available in Your Country
Sorry, this page is not
available in your country.
Overview
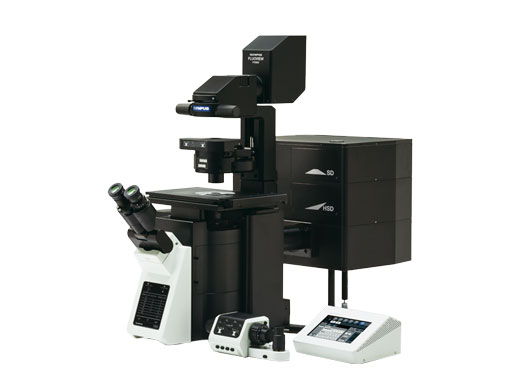
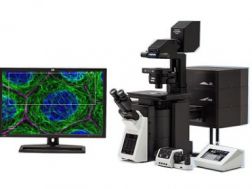
 | Next Generation FLUOVIEW for the Next Revolutions in ScienceThe FLUOVIEW FV3000 series of confocal laser scanning microscopes meets some of the most difficult challenges in modern science. Featuring the high sensitivity and speed required for live cell imaging as well as deep tissue observation, the FV3000 confocal microscope enables a wide range of imaging modalities, including macro-to-micro imaging, super resolution microscopy, and quantitative data analysis. This product has been discontinued, check out our current product |
|---|
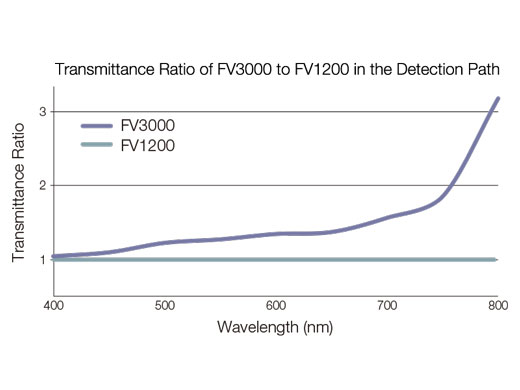
High-Sensitivity Image Multiplexing from Violet to NIRUsing proprietary spectral detection technology, the FV3000 confocal microscope's TruSpectral detectors combine high sensitivity with spectral flexibility to detect even the dimmest fluorophores.
Our new FV3000 Red near-infrared (NIR) solution further extends the FV3000 microscope’s wavelength detection capabilities to the NIR region of up to 890 nm through a suite of carefully engineered NIR upgrades:
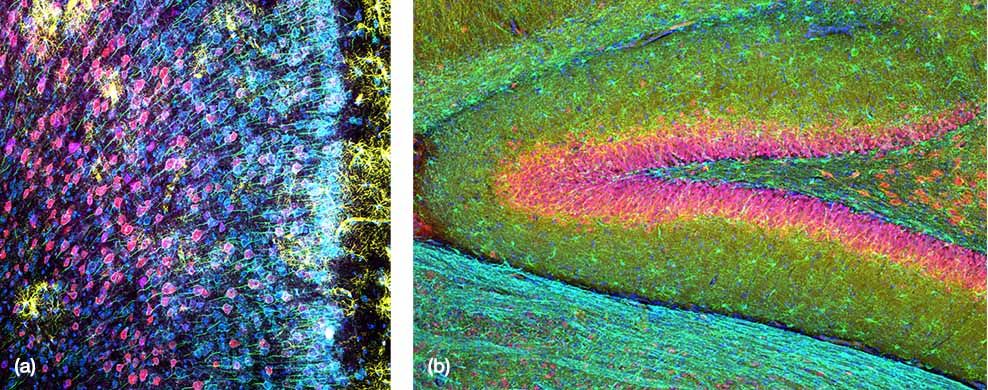
(a) Mouse mPFC labeled with glial fibrillary acidic protein (GFAP; astrocyte marker; yellow), calmodulin-dependent protein kinase II (CaMKII; pyramidal neuron marker; red), amphoterin-induced protein 1 precursor (AMIGO-1; neuronal membrane marker; cyan), parvalbumin (PV; inhibitory neuron marker; purple), ankyrin-G (AnkG; axon initial segment marker; green), and nuclear yellow (nuclei marker; blue).
|
|---|
Macro-to-Micro Imaging and Super Resolution MicroscopyThe FV3000 microscope’s macro-to-micro workflow provides a roadmap for data acquisition, enabling you to see data in context and easily locate regions of interest for higher resolution imaging.
|
Mouse brain hemisection embedded for expansion microscopy (pre-expansion), labeled with secondary antibodies against GFP (Alexa Fluor 488, green), SV2 (Alexa Fluor 565, red), Homer (Alexa Fluor 647, blue). |
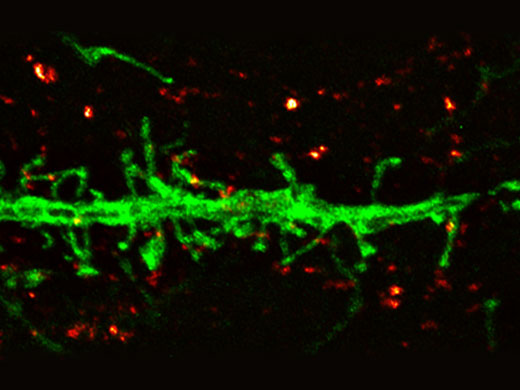
Dendrite (anti-GFP Alexa Fluor 488, green) and synaptic marker (SV2, Alexa Fluor 565, red). Olympus Super Resolution image processed with cellSens advanced constrained iterative deconvolution. Average full width half maximum measurements ~135 nm. Image acquired with 100X 1.35 NA silicone objective. |
Hybrid Scanning for High-Speed Imaging and Increased ProductivityThe FV3000 Hybrid Scanner provides two scanners in one for enhanced confocal imaging capabilities.
| Related Videos |
|---|
Related Videos | Accurate Time-Lapse ImagingTime-lapse experiments require consistent focus and low phototoxicity to the sample.
|
|---|
Deep Tissue Observation with Silicone ObjectivesThe refractive index of silicone oil is close to that of living tissue, enabling high-resolution observation deep inside tissue with minimal spherical aberration.
| 3D image of chick ciliary ganglion cleared by a tissue clearing reagent.
|
|---|
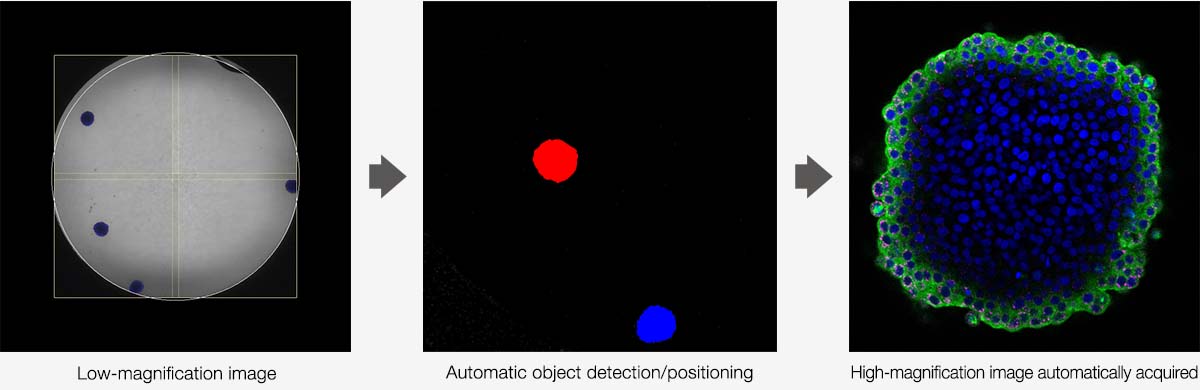
Automated Organoid ImagingFrom finding target objects to capturing high-resolution 3D images, the workflow for imaging organoids can be highly time consuming, particularly when imaging multiple wells across a microplate. The Macro-to-Micro module for the FV3000 system is a smart solution that automates the organoid imaging workflow to dramatically increase imaging efficiency. The FV3000 microscope captures images at low magnification, and then the Macro-to-Micro software module can automatically locate your objects of interest in the vessel or well and capture them at high magnification. This automated process dramatically reduces the time you spend on microscope operation. |
|---|
|
Applied Technologies
TruSpectral DetectionGroundbreaking TruSpectral detection technology offers outstanding results compared to previous generations of spectral detection units. Featured on every channel in the FV3000 microscope, TruSpectral detection technology combines the flexibility of a spectral detector with the sensitivity of a filter-based detector. |
|
| |
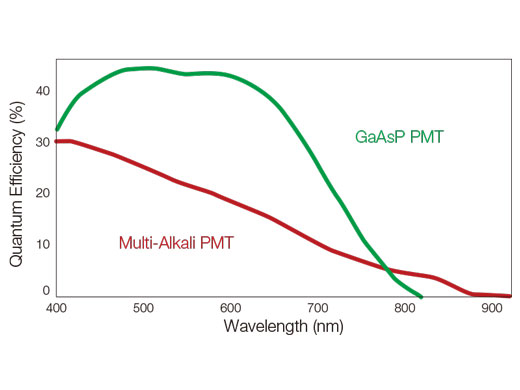
How TruSpectral Detection Technology WorksBased on patented volume phase hologram (VPH) transmission for high efficiency, TruSpectal detection uses an adjustable slit to select the detection wavelengths of each individual channel to 2 nm. | Sensitivity and AccuracyIntegrated in all FV3000 confocal microscopes, TruSpectral detection technology enables much higher light throughput compared to conventional spectral detection units. The volume phase hologram diffracts light with up to three-fold higher transmission efficiency vs. reflection gratings. The result is excellent multicolor fluorescence microscopy images of live and fixed tissue. | Enhanced Quantum EfficiencyThe FV3000 microscope's high-sensitivity detector (HSD) enables you to view samples whose emission is too weak to view with conventional detectors. The HSD unit incorporates two GaAsP channels with a maximum quantum efficiency of 45% and Peltier cooling that reduces background noise by 20% for high signal-to-noise (S/N) ratio images under very low excitation light. HSD units can be combined to enable four-channel GaAsP imaging with the FV3000 system. |
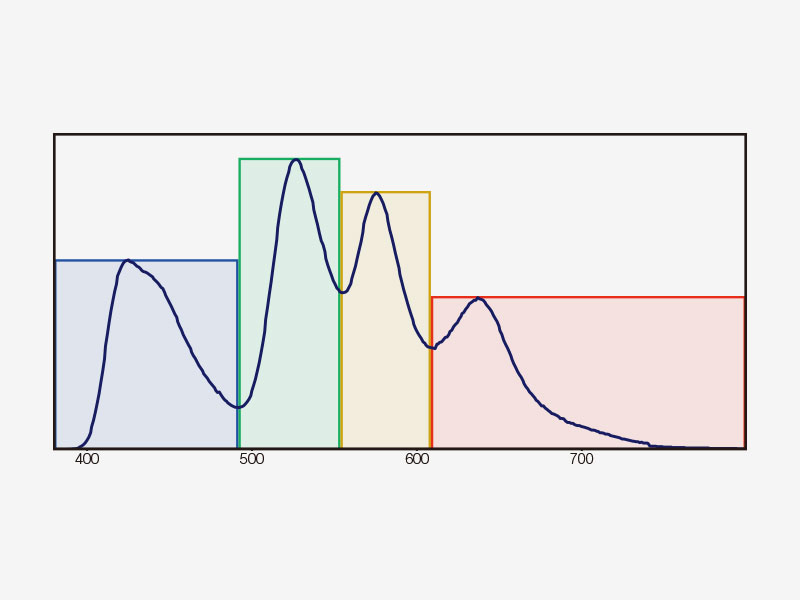 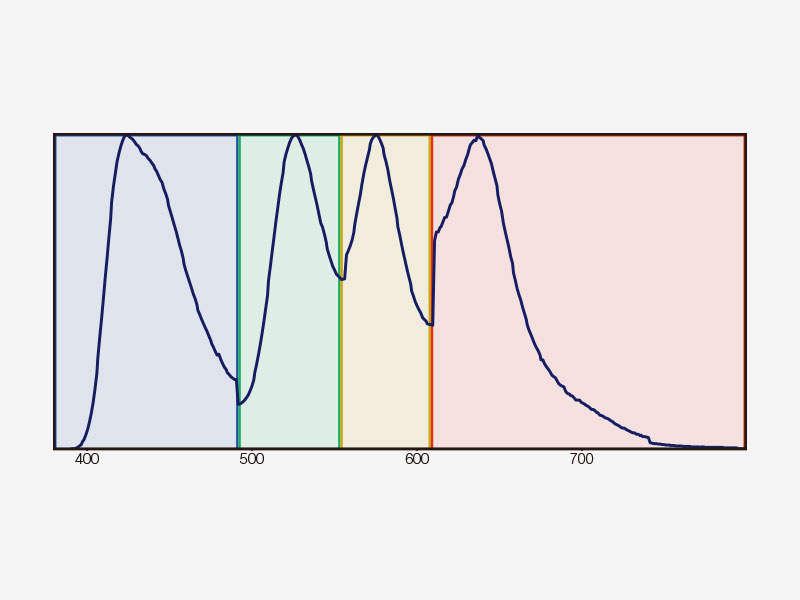 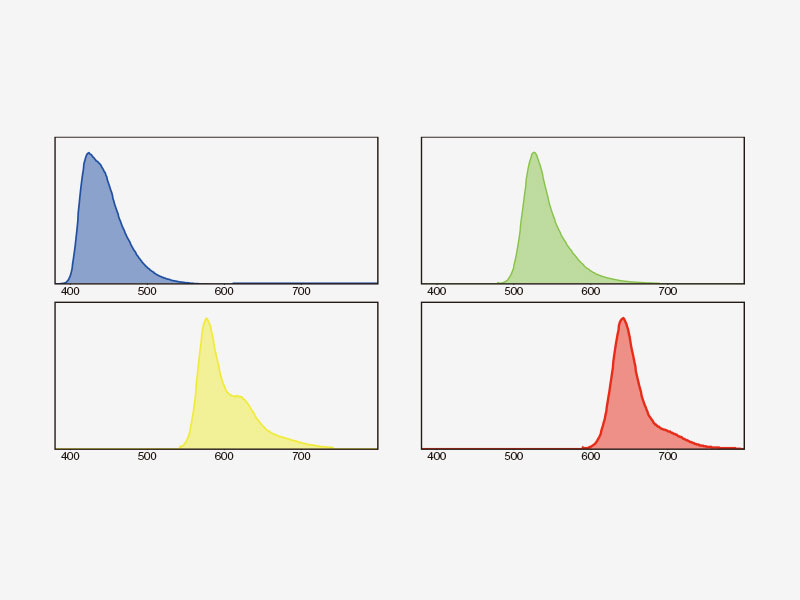 | Multichannel TruSpectral Detection with Sixteen-Channel UnmixingTruSpectral detection works independently on all microscope channels, enabling true simultaneous multichannel lambda scanning on up to four channels. Multichannel lambda mode facilitates both live and post-processing spectral unmixing for excellent spectral separation results. With up to four dynamic ranges, bright and dim signals can be optimally separated by independently adjusting the sensitivity of each detector. |
|---|
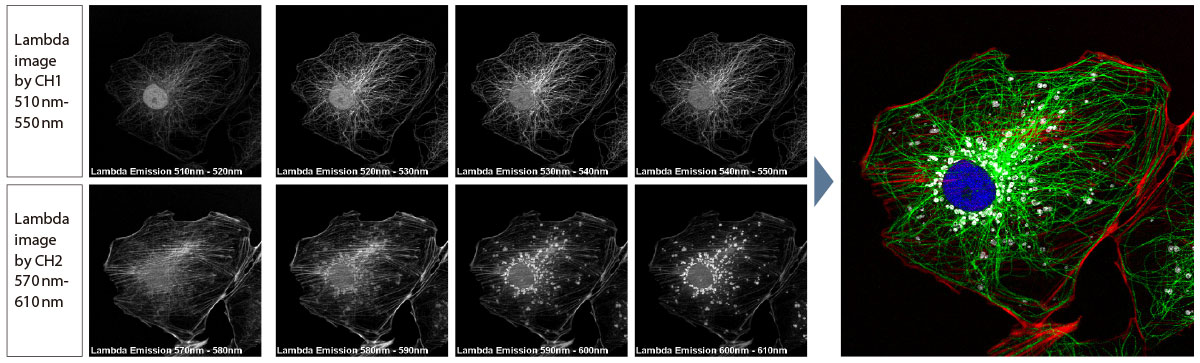
Spectral UnmixingThe FV3000 system’s spectral deconvolution algorithm enables overlapping spectra to be separated based on the spectral information from lambda stack images. Fluorescence cross-talk between channels can be eliminated by the unmixing algorithm during both live image acquisition and post-processing, enabling clean separation of up to sixteen fluorophores. |
Spectral unmixing of a PtK2 cell labeled with YOYO-1, Alexa Fluor 488, Rhodamine-phalloidin, and MitoTracker Red using multi-channel lambda stack image. |
Platelets bound to a thrombosis in the blood vessel of a mouse. Images taken at 30 fps in full frame by a resonant scanner with 2 CH GaAsP PMTs.
| Two Scanner OptionsChoose between two scan units: a traditional galvanometer scanner (FV3000) or a hybrid galvanometer/resonant scanner (FV3000RS).
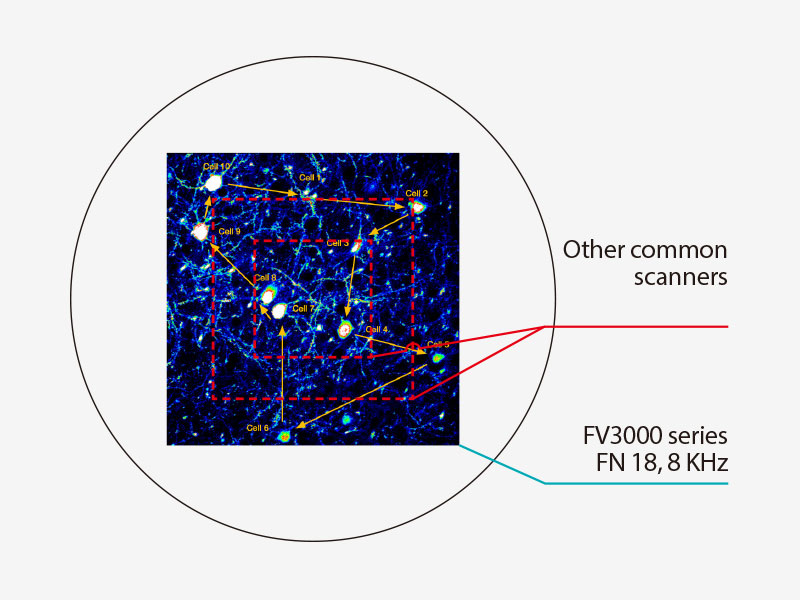
No Compromise Between Speed and Field of ViewMany high-speed scanning methods restrict the field of view, limiting your ability to examine large areas with multiple cells. The resonant scanner in the FV3000RS microscope maintains a full 1X field of view with FN 18, even at a video rate of 30 frames per second. Clipping the Y axis enables speeds up to 438 frames per second. |
|---|
Rolling Average ProcessingWhile high-speed scanning with low laser power minimizes phototoxicity, it often decreases the signal-to-noise ratio, making it difficult to obtain high-resolution time-lapse images. With rolling average processing, you can adjust high-speed time-lapse images to achieve better signal-to-noise ratios while maintaining the time scale and keeping the original data. |
(Right) Rolling average processing (10 frames) on 30 fps data acquired at low laser power. |
|---|
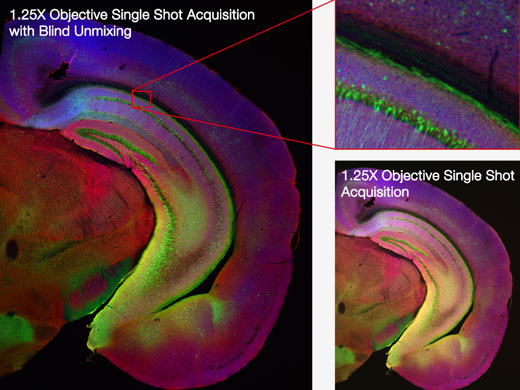
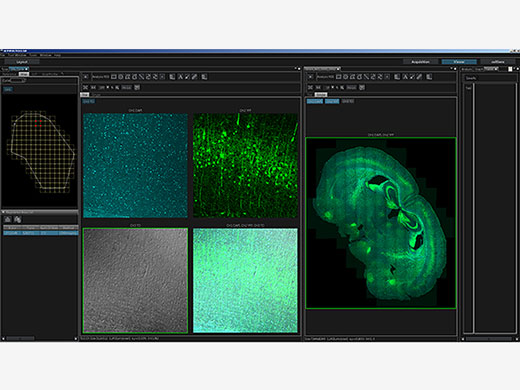 | Macro-to-Micro ObservationSee data in context with macro-to-micro observation. With the FV3000 system’s redesigned light path, generate detailed overview images with magnifications as low as 1.25X, then easily identify structures to image at higher magnification. Mosaic imaging enables you to acquire continuous 3D (XYZ) and 4D (XYZT) images of adjacent fields of view. The entire process from image acquisition to stitching can be fully automated, saving time and generating more meaningful data. |
|---|
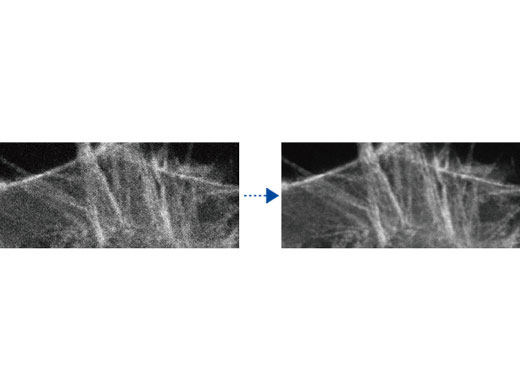
TruSight Deconvolution: Image Processing for Higher ResolutionRemove blur and obtain clearer, sharper images with TruSight deconvolution. Specialized cellSens algorithms for the FV3000 confocal microscope enable a seamless workflow from acquisition to publication with the click of a button. Take advantage of GPU processing for even faster results. | 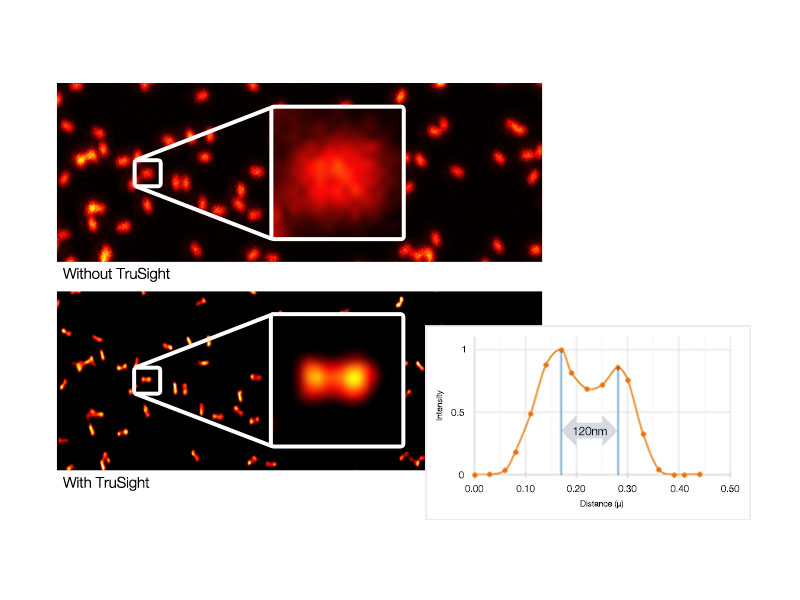  Left: Raw confocal image / Right: Image with TruSight |
|---|
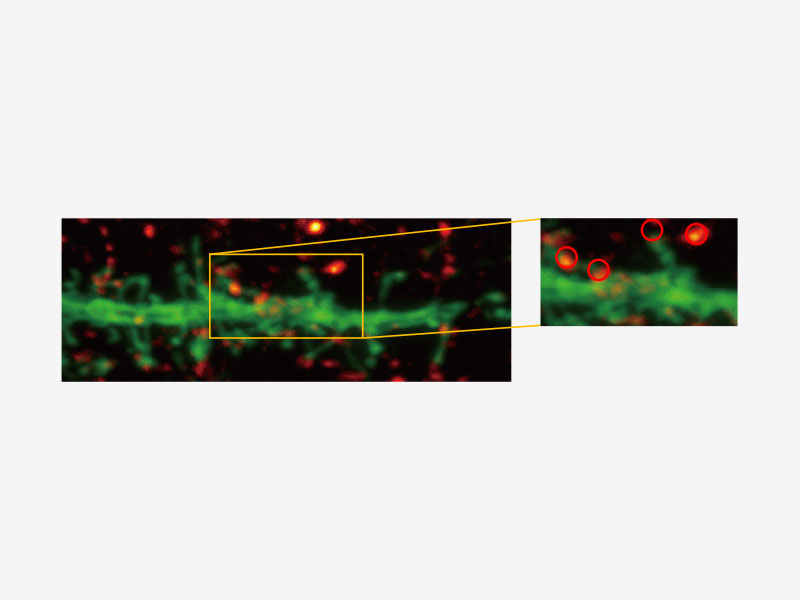 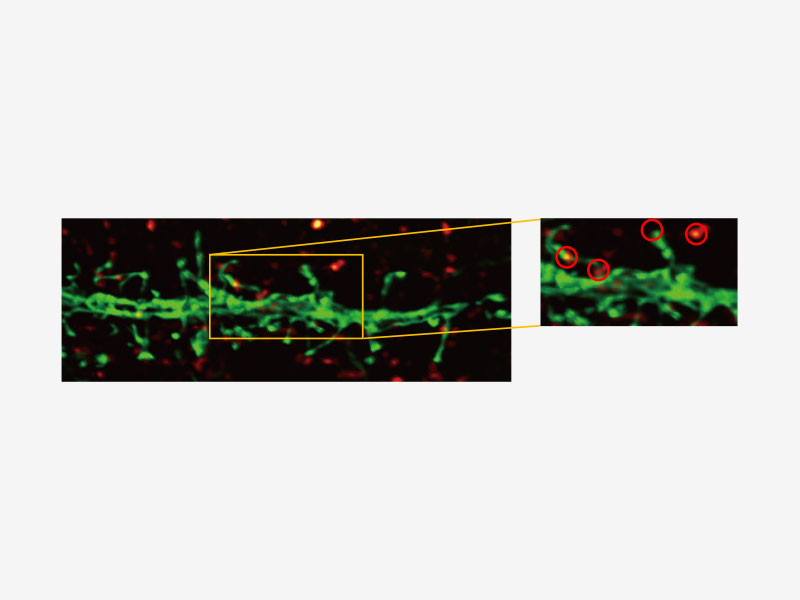 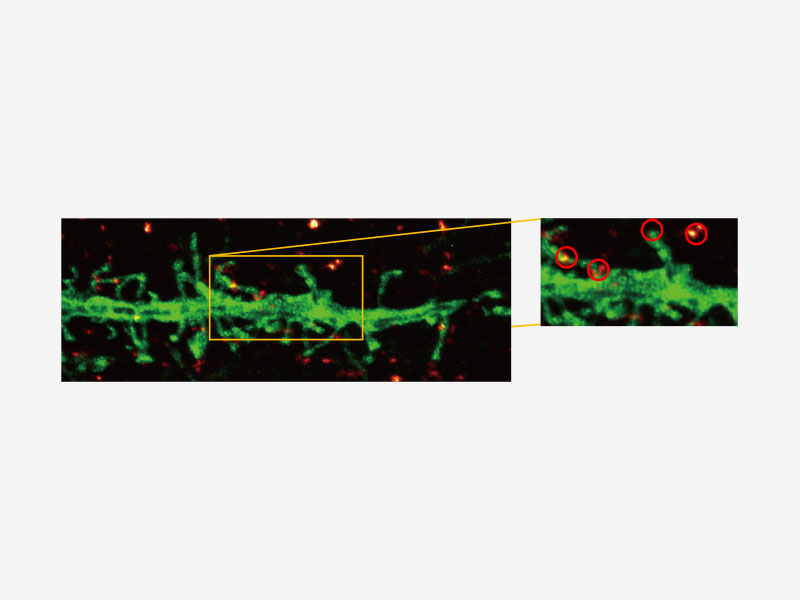 | Olympus Super Resolution (FV-OSR) Technology with Up to Four Simultaneous ChannelsSuitable for colocalization analysis, the Olympus Super Resolution imaging module can acquire four fluorescent signals either sequentially or simultaneously with a resolution of approximately 120 nm, nearly doubling the resolution of typical confocal microscopy.
Learn more about the FV3000 Super Resolution Software Module |
|---|
TruFocus Time-Lapse ImagingTruFocus technology makes multi-position experiments and long-term time-lapse imaging more robust and reliable. TruFocus technology uses a minimally phototoxic infrared laser (Class 1) to identify the location of the sample plane and offers two modes for maintaining focus.
| Related Videos |
|---|
 Conventional objectives (left) versus X Line objectives (right) | High-Performance Microscopic Imaging with X Line ObjectivesProviding broad chromatic aberration correction, uniform images, and a high numerical aperture, X Line objectives enhance the FV3000 confocal microscope’s image quality. |
|---|
Deep Tissue Observation with Silicone Immersion ObjectivesOlympus offers four high-NA silicone immersion objectives that deliver excellent performance for live cell imaging.
| Related Videos |
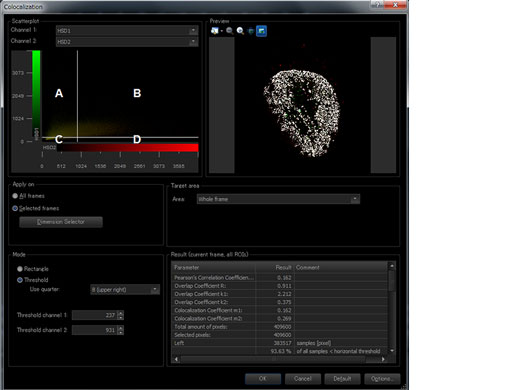
 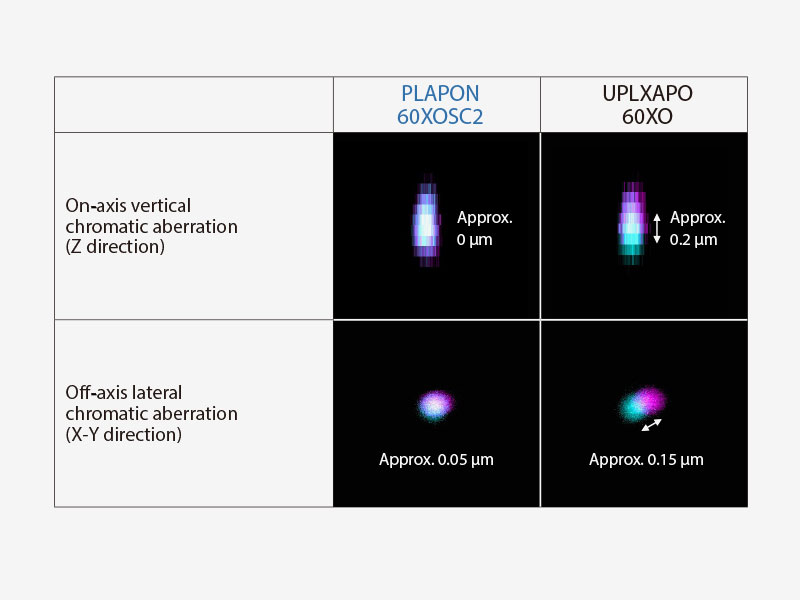 | Enhance the Reliability of Colocalization Analysis with a Low Chromatic Aberration Objective (PLAPON60XOSC2)This oil immersion objective minimizes lateral and axial chromatic aberration in the 405–650 nm spectrum, enabling you to acquire reliable colocalization images and measure objects with positional accuracy. The objective also compensates for chromatic aberration through near infrared (up to 850 nm), making it ideal for quantitative microscopy imaging.
|
|---|
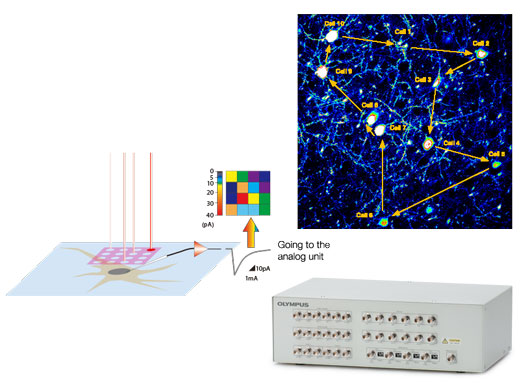
Optimized Configurations for Electrophysiological ExperimentsSynchronize confocal imaging events with electrophysiology equipment via the trigger signal I/O interface box. The I/O interface box also converts voltage signals into images that can be treated in the same manner as fluorescence images. This enables you to capture voltage-based images in synchrony with photostimulation by the confocal scanner. |
Software
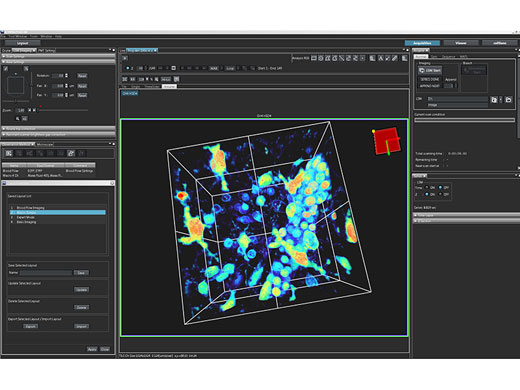
Intuitive SoftwareThe FV3000 software streamlines your entire confocal imaging workflow, from acquisition to analysis. Customizable and savable layouts make it easy to tailor the interface to your workflow and experiment needs, no matter the level of complexity. |
|
|---|
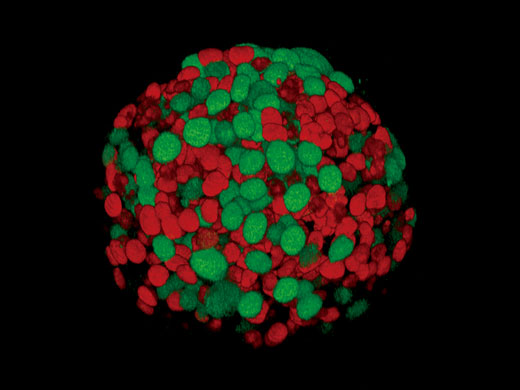
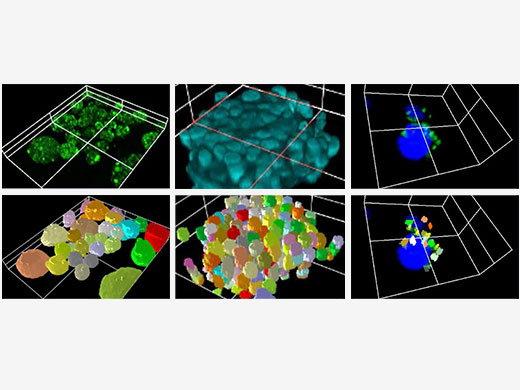
Live 3D Rendering and AnalysisSee your data unfold in real time with the FV3000 software’s live 3D image display function. 3D images can be constructed during image acquisition and shown as live images. Additional 3D cell analysis is available using optional NoviSight software. Take advantage of the software’s powerful capabilities to quantify spheroid cells or other 3D objects in microplate-based experiments. |
Fucci induced spheroid of the HT29 cell line
|
Various 3D cell analyses are available with optional NoviSight software. |
Multi-Area Time-Lapse and Microplate ImagingThe Multi-Area Time-Lapse (MATL) module provides robust and accurate time-lapse data through precise control over motorized stage movements, enabling you to generate detailed overview microscope images to see data in context. Pair the MATL module with the well navigator module to achieve more functionality with sophisticated, intuitive controls for a wide range of cell culture vessels and custom microplates. |
|
|---|
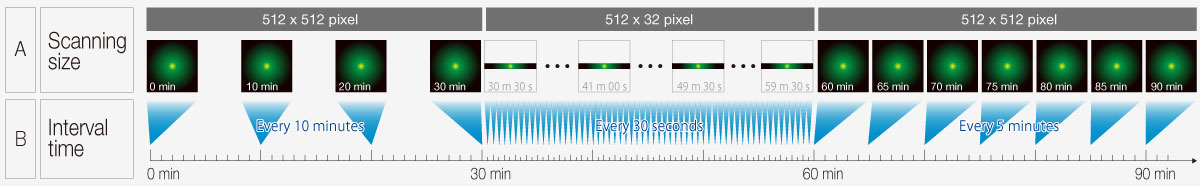
Reduce Complexity with the Sequence ManagerThe Sequence Manager software module handles complex protocols with ease. Multiday time-lapse experiments are controlled with microsecond scan accuracy and millisecond sequence execution accuracy. Various complex protocols can be performed, including:
|
 |
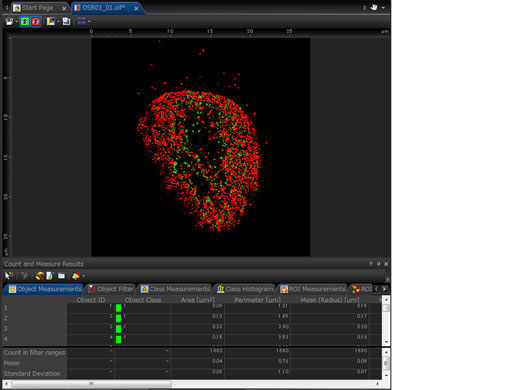
Quantitative Microscopy Image AnalysisThe FV3000 confocal microscope incorporates a suite of optional analysis functions to complete your imaging workflow and provide quantitative data.
|
Count and Measure
|
|
FRET and FRAP ExperimentsThe FV3000 microscope paired with the cellSens Life Science Analysis module enables easy acquisition and analysis of FRET and FRAP experiments.
|
| 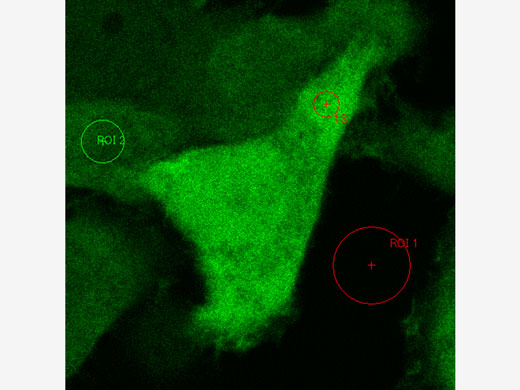 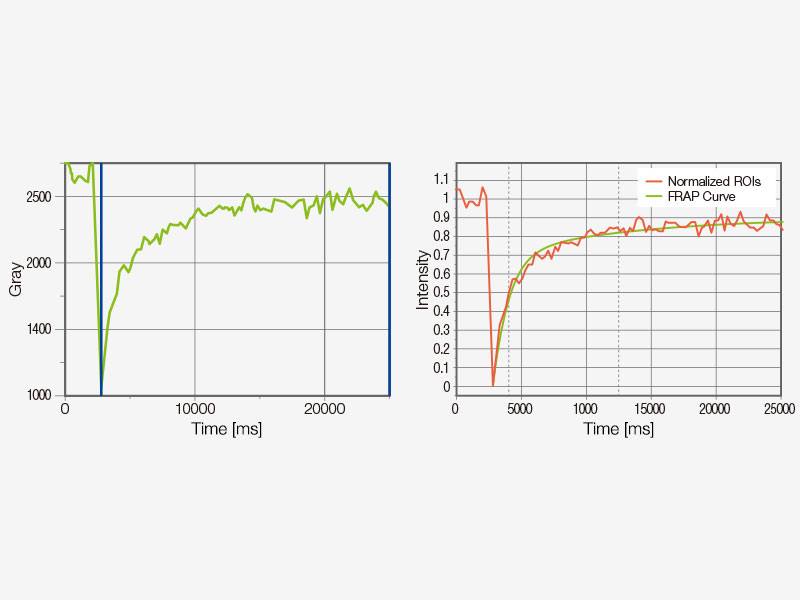 |
Example of FRET analysis (acceptor photobleaching) | Example of FRAP analysis |
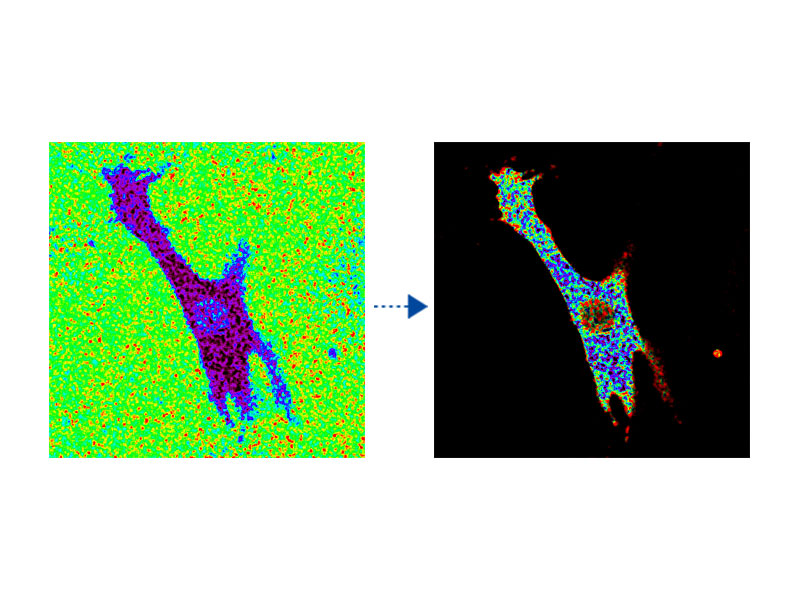
Ratio Imaging and Intensity Modulated Display (IMD)The FV3000 microscope's ratio imaging analysis software includes an Intensity Modulated Display (IMD) function that displays quantitative fluorescence ratio changes during both standard and high-speed acquisitions. This function is particularly useful for calcium and FRET imaging where a pure ratio display provides poor contrast in background areas. |
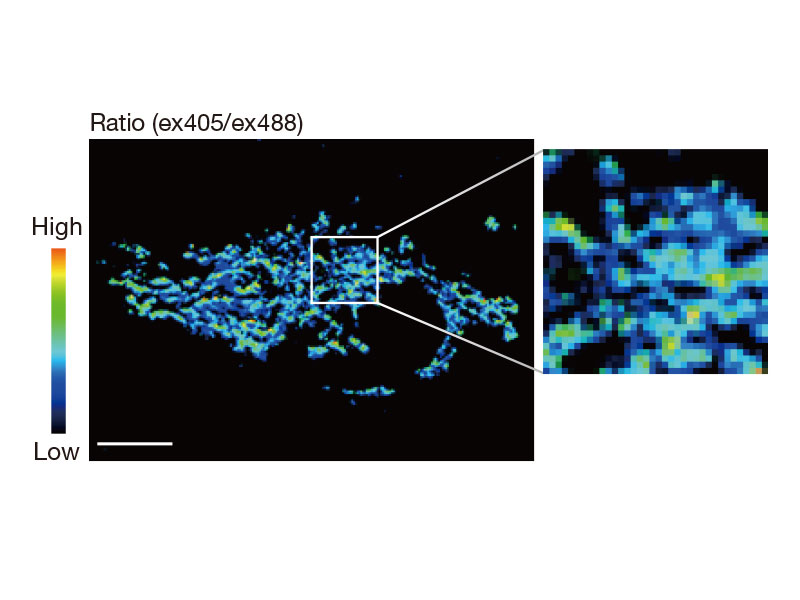 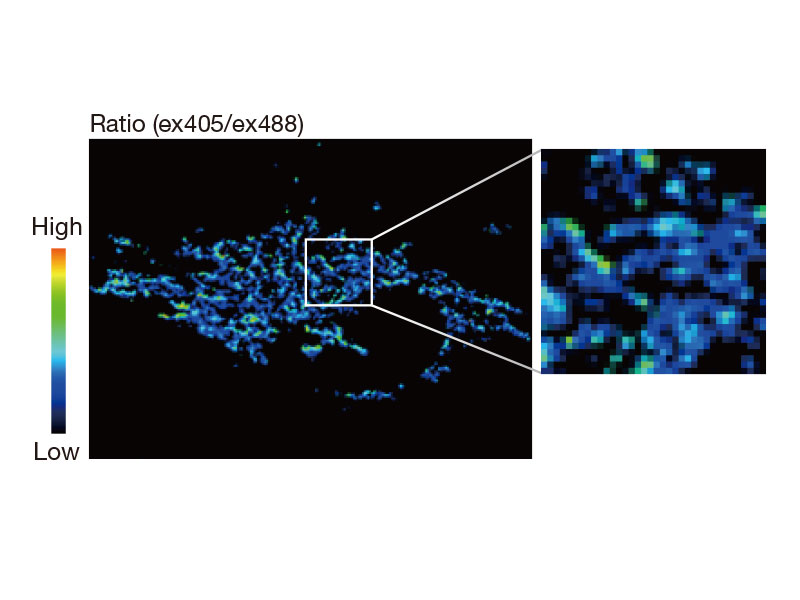
tsGFP1-mito reveals heterogeneity in mitochondrial thermogenesis in HeLa cells.
|  
Cardiomyoctye
|
Bioluminescence of RA-induced differentiating cells at day 12 from Bmal1:luc stably transfected ES cells
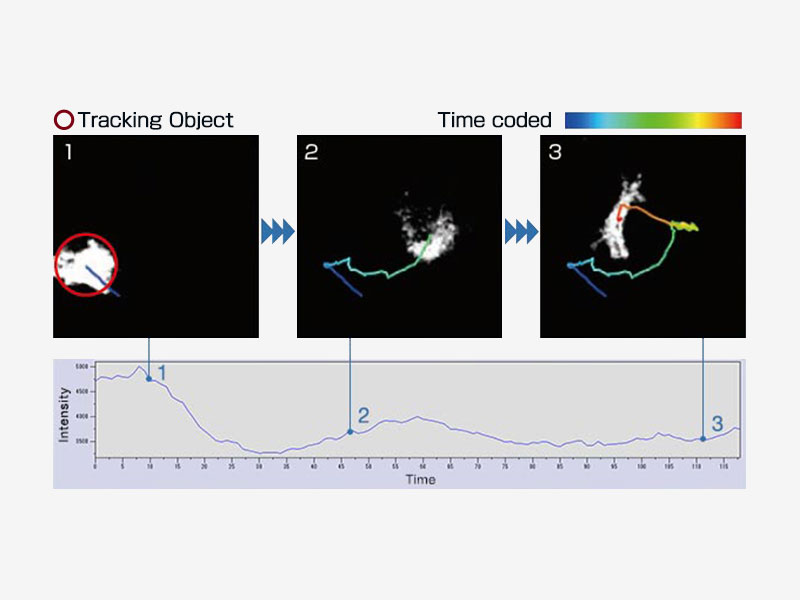
| Object TrackingAutomatically detect, track, and analyze moving objects in time-lapse images using the cellSens Object Tracking module. The tracking function provides a powerful and intuitive tool to quantify dynamic processes, such as cell movement and division. |
|---|
Remote Development Kit (RDK) ModuleThe Remote Development Kit enables remote control and programming of certain FV3000 microscope functions in languages like Python, C++, and Matlab. The RDK module is a powerful tool for users with programming experience to unlock even greater potential behind the system. | 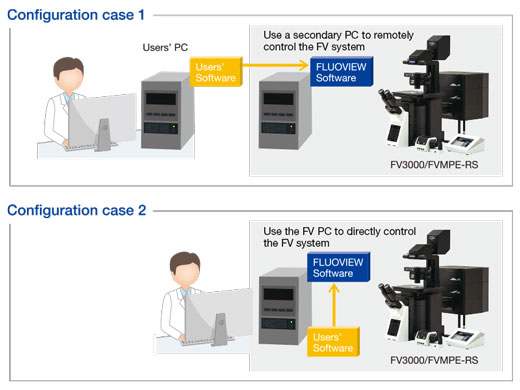 |
|---|
Configurations
Choose the Configuration that Suits Your Application |
Inverted microscope
|
Upright microscope (configured for slide imaging)
|
Upright microscope (configured for electrophysiology)
|
Components FV3000 Main Laser Combiner and Sub Laser Combiner
FV3000 Main Laser Combiner and Sub Laser CombinerFLUOVIEW Laser Scanning Microscope Solutions Software FV3000 Multi Area Time Lapse Software Module
FV3000 Multi Area Time Lapse Software ModuleFLUOVIEW laser scanning microscope solutions FV3000 Multi Point and Mapping Software ModuleFLUOVIEW Laser Scanning Microscope Solutions 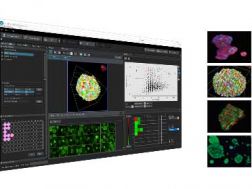 NoviSight NoviSight 3D cell analysis software provides statistical data for spheroids and 3D objects in microplate-based experiments. Use it to quantify cell activity in 3D, easily capture rare cell events, obtain accurate cell counts, and improve detection sensitivity. NoviSight software works with a range of imaging techniques, including point-scan confocal imaging, two-photon imaging, spinning disk confocal imaging, and super resolution live cell imaging.
Scanners |
Specifications
| FV3000 | FV3000RS | ||
|---|---|---|---|
| Main Laser Combiner | Visible Light Laser |
405 nm: 50 mW, 488 nm: 20 mW, 561 nm: 20 mW, 640 nm: 40 mW
One optional laser port for the sub laser combiner or optional laser unit | |
| Optional Laser | Sub Laser Combiner |
Maximum 3 laser units as follows:
445 nm: 75 mW, 514 nm: 40 mW, 594 nm: 20 mW, fiber connected to main laser combiner | |
| Single Laser Unit | 445 nm: 75 mW, 514 nm: 40 mW, or 594 nm: 20 mW, directly connected to main laser combiner | ||
| Single NIR Laser Unit | 730 nm: 30 mW, 785 nm: 100 mW, connected to scanner via optional port | ||
| Scanner | Scanning Method | 2 silver-coated galvanometer scanning mirrors |
2 silver-coated galvanometer scanning mirrors
1 silver-coated resonant and 1 silver-coated galvanometer scanning mirrors |
|
Galvanometer Scanner
(Normal Imaging) |
Scanning Resolution: 64 × 64 to 4096 × 4096 pixels
Scanning Speed (One Way): 512 × 512 with 1.1 s–264 s. pixel time: 2 µs–1000 µs Scanning Speed (Round Trip): 512 × 512 with 63 ms–250 ms, 256 × 256 with 16 ms–125 ms Optical Zoom: 1X–50X in 0.01X increments Scan Rotation: Free rotation (360 degree) in steps of 0.1 degree Scanning Mode: PT, XT, XZ, XY, XZT, XYT, XYZ, XYλ, XYZT, XYλT, XYλZ, XYλZT ROI scanning, rectangle clip, ellipse, polygon, free area, line, free line and point, tornado mode only for stimulation | ||
|
Resonant Scanner
(High-Speed Imaging) | - |
Scanning Resolution: 512 × 32 to 512 × 512 pixels
Scanning Speed: 30 fps at 512 × 512, 438 fps at 512 x 32 Optical Zoom: 1X–8X in 0.01X increments Scanning Mode: XT, XZ, XY, XZT, XYT, XYZ, XYλ, XYZT, XYλT, XYZ, XYλZT ROI scanning, rectangle clip, line | |
| Pinhole | Single motorized pinhole, pinhole diameter ø50–800 µm (1 µm steps) | ||
| Field Number (FN) | 18 | ||
| Dichromatic Mirror Turret | 8 positions (high-performance DMs and 10/90 mirror) | ||
| Optional Unit for Scanner | Laser power monitor, optional laser port | ||
| Spectral Detector | Detector Module | Cooled GaAsP photomultiplier (high-sensitivity type) or Multi-Alkali photomultiplier, 2 channels | |
| Spectral Method |
Motorized volume phase holographic transmission diffraction grating, motorized adjustable slit,
selectable wavelength bandwidth: 1–100 nm, wavelength resolution: 2 nm | ||
| Dichromatic Mirror Turret | 8 positions (high-performance DMs and mirror) | ||
| NIR Detector | Detector Module | GaAs photomultiplier tube, 1 channel or 2 channels with filter cube | |
| Fluorescence Illumination Unit | External fluorescence light source, fiber adaptor to the optical port of the scan unit, motorized switching between the LSM light path and fluorescence illumination | ||
| Transmitted Light Detector Unit | Module with integrated external transmitted light photomultiplier detector and LED lamp, motorized switching | ||
Microscope |
| Inverted frame | Upright frame (for imaging) | Upright frame (for electrophysiology) | |
|---|---|---|---|
| Microscope Frame |
Motorized inverted microscope
IX83 (IX83P2ZF) |
Motorized fixed stage upright microscope
BX63L |
Motorized fixed stage upright microscope
BX63L |
| Revolving Nosepiece | Motorized sextuple revolving nosepiece | Motorized septuple revolving nosepiece |
Coded swing nosepiece
Coded slider nosepiece |
| Condenser | Motorized long working distance condenser | Motorized universal condenser | Manual long working distance condenser |
| Focus Stroke |
Built-in motorized nosepiece focus
Stroke: minimum increment: 0.01 μm | ||
Software |
| Basic Features |
GUI designed for darkroom environment. User-arrangeable layout.
Acquisition parameter reload features. Hard disk recording capability; adjust laser power and HV with Z-stack acquisition. Z-stack with alpha blending, maximum-intensity projection, iso-surface rendering. |
|---|---|
| 2D Image Display | Each image display: single-channel side-by-side, merge, cropping, live tiling, live tile, series (Z/T/λ), LUT: individual color setting, pseudo-color, comment: graphic and text input. |
| 3D Visualization and Observation |
Interactive volume rendering: volume rendering display, projection display, animation displayed.
3D animation (maximum intensity projection method, α blending) 3D and 2D sequential operation function. |
| Image Format |
OIR image format
8/16-bit gray scale/index color, 24/ 32/ 48-bit color, JPEG/ BMP/ TIFF image functions, Olympus multi-tif format. |
| Spectral Unmixing | Fluorescence spectral unmixing modes (up to 16 channels) |
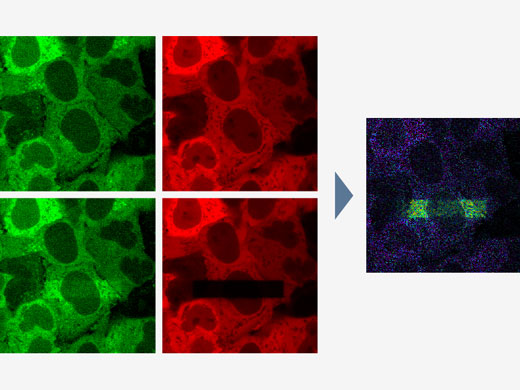
| Image Analysis | Region and line measurements, intensity plot over time/Z, colocalization analysis. |
| Statistical Processing | 2D data histogram display. |
| Optional Software | Motorized-stage control |