Actin filaments in animal cells are organized into two different styles: bundles and weblike (gel-like) networks. Actin filament cross-linking proteins are accordingly divided into two classes: bundling proteins and gel-forming proteins. Bundling proteins cross-link actin filaments into a parallel array, while gel-forming proteins hold two actin filaments together at a wide angle to each other, creating a looser framework. In the digital video presented above, embryonic rat thoracic aorta fibroblast cells (A7r5 line) are expressing a fusion of Dronpa fluorescent protein to human beta-actin.
Video 1 - Run Time: 12 Seconds
Video 2 - Run Time: 06 Seconds
Video 3 - Run Time: 31 Seconds
Video 4 - Run Time: 09 Seconds
Video 5 - Run Time: 09 Seconds
Video 6 - Run Time: 09 Seconds
Video 7 - Run Time: 07 Seconds
Video 8 - Run Time: 12 Seconds
Video 9 - Run Time: 17 Seconds
The actin strands in muscle are made up of many smaller globular subunits assembled in a long chain. Each filament is composed primarily of two such actin chains wound around each other. The myosin molecule has the longest protein chains known, each one consisting of some 1,800 amino acid units. Each long chain has a globular structure at one end. These globular heads have two crucial functions—they are the binding sites that link the actin and myosin molecules, and they act as enzymes to split ATP to ADP, thus providing the energy for muscle contraction. In the digital video presented above, embryonic rat thoracic aorta fibroblast cells (A7r5 line) are expressing a fusion of Dronpa fluorescent protein to human beta-actin.
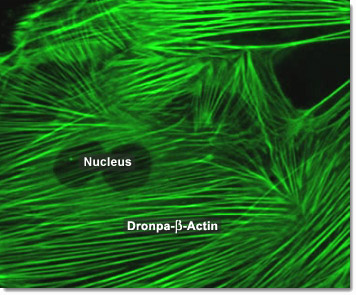
Actin filaments in animal cells are central to the process of cleavage which leads to cytokinesis. The first sign of cleavage is the appearance of a furrow, a shallow groove in the cell surface. On the cytoplasmic side of the furrow is a contractile ring of actin microfilaments associated with molecules of the protein myosin. The actin microfilaments interact with the myosin molecules, causing the ring to contract. The cleavage furrow deepens until the parent cell is pinched in two, producing two completely separated cells, each with its own nucleus and share of cytosol and organelles. In the digital video presented above, embryonic rat thoracic aorta fibroblast cells (A7r5 line) are expressing a fusion of Dronpa fluorescent protein to human beta-actin.