このページはお住まいの地域ではご覧いただくことはできません。
特長
 | Olympus cellFRAP is designed with researchers in mind, providing highly accurate and flexible live-cell photomanipulation with various evaluation options for data presentation needs. cellFRAP employs a diffraction-limited laser spot for high-intensity and precise positioning of the bleaching region across the full field of view. The systems’ excellent spot quality allows full flexibility in position and shape of the bleaching region, and enables control of the bleaching area with extreme accuracy. Via incorporation into the Olympus IX3 open source frames, the cellFRAP deck can easily be adapted to an existing imaging platform with a full range of compatible accessories. Using the cellFRAP solution in the Olympus cellSens Graphical Experiment Manager provides simple system set-up and experiment control. |
|---|
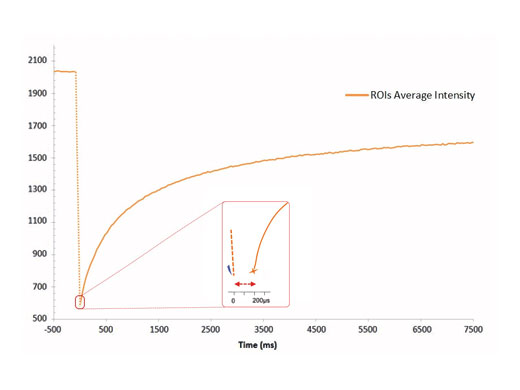
Highest Spatial and Temporal ResolutionThe cellFRAP system guarantees highest spatial and temporal resolution for acquiring reliable and reproducible data in photomanipulation applications. Based on the system’s diffraction-limited spot properties, bleaching areas can be controlled extremely accurately. Driven by the Olympus Real-time controller, the cellFRAP system offers an unrivaled short delay time of only 200 µs between bleach and postbleach acquisition. Therefore, the system enables observation and collection of all important and immediate sample responses. |
200 µs between bleaching and acquisition |
A diffraction-limited spot size guarantees scanning precision and uniformity across the overall scan area |
Diffraction-Limited Laser SpotThe Olympus cellFRAP solution enables the best photomanipulation results without the need to move the sample. By utilizing a diffraction-limited laser spot, cellFRAP does not only guarantee the highest bleaching intensity but also ensures a precise and flexible choice in shape and position of the bleaching regions across the full field of view. High-quality chromatically corrected optics open up the possibility of using up to four different lasers during one experiment—for example, for optogenetics applications. |
ReproducibilityUltraprecise experimental control and reproducibility are of interest to most users. The cellFRAP system accounts for these demands by ultraprecise experimental control given by high system-level synchronization at ~1 µs temporal resolution. In addition, the complete workflow of an experiment is documented and can easily reproduced at any time. |
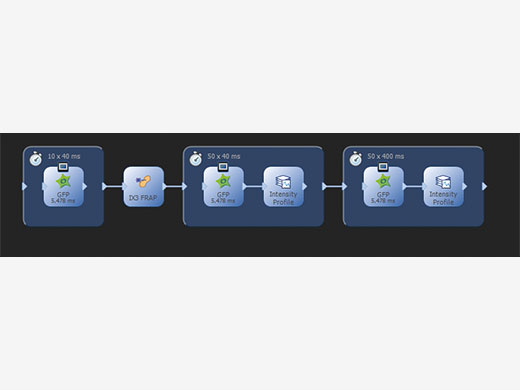
Straight Forward Setup and ControlWorking with cellFRAP is intuitive and simple as the system is completely integrated into cellSens, the Olympus Life Science imaging platform. An automated calibration procedure makes it easy to perfectly align the scanner with the camera while taking into account the objectives and laser lines in use. Operating cellFRAP in the cellSens Graphical Experiment Manager offers intuitive system settings and experiment control, and it allows utmost operational flexibility and optimizes for the best speed performance. Thus, even complex acquisition workflows can be easily defined and reproduced, and any device operation can be automated. |
Using the GEM (Graphical Experiment Manager) of cellSens, even complex acquisition and analysis workflows are defined and easily repeated |
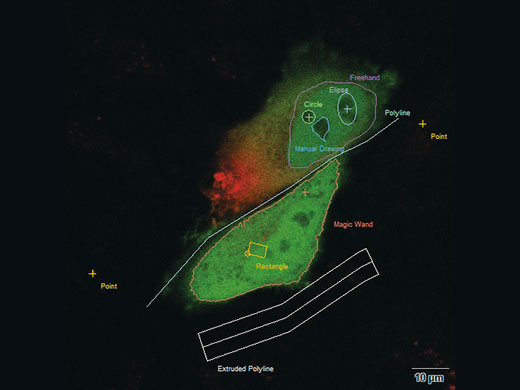
You can bleach points, lines or areas in variable shapes and modify your bleach conditions independently and separately for each shape |
|
Interactive Experiment ControlcellFRAP makes it possible to precisely adapt the bleaching regions to sample structures. An interactive definition of experimental conditions includes a free choice of bleaching/photomanipulation regions—variable in shape, including points and lines. Two different operation modes are available: Manipulation either based on predefined regions of interest or via interactive “Click & Bleach” mode. Signal intensity changes over time can also be observed online by displaying an intensity profile on demand. |
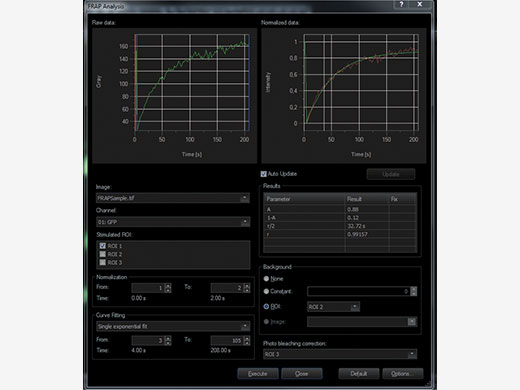
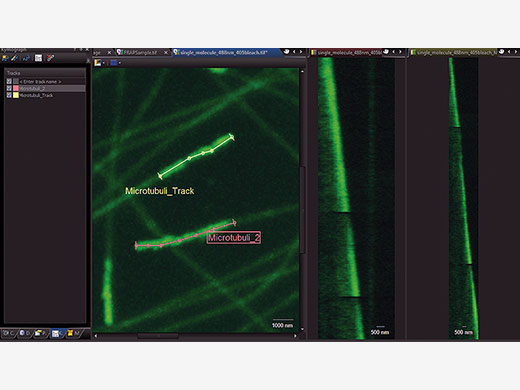
Choose Your Method of Evaluating DataIn order to accurately evaluate your data, time markers for the start and end of the photomanipulation event are added to the recorded data. cellFRAP includes a qualitative FRAP analysis to classify your results. Here, recovery rates and mobile and immobile fractions can quickly be calculated from the recorded data including background and bleaching corrections. In addition, a Kymograph resembles a timeline visualization along a user-defined trajectory. An integrated export function allows for further evaluation of the data. |
|
Timeline visualization for easy interpretation of data and representation in publications |
Flexibility According to Your NeedsIntegrated within the Olympus IX3 open-source concept, the cellFRAP deck solution can easily be adapted to an existing imaging platform and combines with a wide range of accessories like ZDC, climate chambers, manual or motorized stages, manipulators, etc. This allows for the setup of photomanipulation experiments in combination with other high-end applications—for example, spinning-disk and TIRF microscopy. The system can be equipped with up to four lasers for photomanipulation, a prerequisite for further sophisticated applications such as distinct photoswitching or optogenetics. |
Need assistance? |
アプリケーション
Photoactivation and PhotoswitchingMany cellular particles move quickly and unpre-dictably—for example, vesicles or intracellular pathogens such as viruses. For this reason, fluorescent molecules exhibiting photoactivation and photoconversion properties are used as markers to track the dynamic behavior. For example, fluorophores are available that can be changed-reversible or irreversible-in their spectral characteristics upon illumination with specific wavelengths—for example PA-GFP, KFP1, and Kaede. In particular, following the fate of, for example, a protein by an activated tagged molecule without the need to constantly providing addional fluorophores provides clear signals and minimal background conditions. |
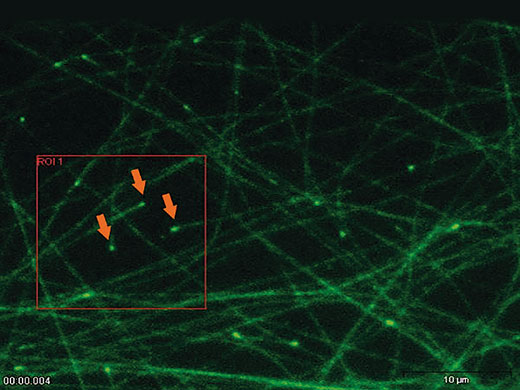
‘Click & Bleach’The ‘Click & Bleach’ function in the cellSens software enables the user to react to sample dynamics and to photomanipulate a target using direct mouse interaction. Multiple points can be bleached or activated and the resultant images can be captured during, or immediately after, the bleaching procedure. |
FRAPFluorescence recovery after photobleaching (FRAP) provides an ideal method for calculating the coefficient of diffusion of a particular molecule within a certain target area of the cell. This is achieved by photobleaching the fluorophore attached to a specific molecule within the target area and then assessing the dynamics of the return of fluorescence (recovery) to that area. |
FLIPFluorescence loss in photobleaching (FLIP) is useful for studying molecular movement along cell membranes (lateral membrane fluidity) and membrane continuity, especially for membranous organelles. A defined area of the membrane or cell is continuously bleached, and the dynamic loss of fluorescence from the remaining area is measured based on the replacement of the bleached molecules in the defined area by unbleached ones from the remainder. |
FLAPFluorescence localization after photobleaching (FLAP) is a clever adaptation of FRAP where the dynamics of a particular molecular species are of interest. The target molecule is coexpressed with two different fluorophores such that distribution and localization overlap. One of the fluorophores is then bleached at a defined location, and the movement of the molecule can be followed by comparing the relative and absolute distribution of the two fluorophores. |
Need assistance? |