Using silicone oil immersion objectives with a confocal laser scanning microscope for deep tissue observation in cleared specimens
Deep tissue observation using a clearing technique that renders specimens transparent
One of the first studies involving the use of "Scale," a clearing technique for rendering biospecimens transparent, was published in the August 2011 issue of Nature Neuroscience.* Since then, various techniques for rendering samples transparent, such as SeeDB, Clarity, ScaleS, and Clear See, have been developed. These techniques are used in conjunction with a two-photon microscope to observe inside tissues to a depth of 8 mm. Currently, two-photon microscopes are only available at a limited number of research institutes, so new methods to observe cleared specimens using widely available laser confocal microscopes were developed.
The choice of objective lens is important when using a laser confocal microscope for deep tissue observation of cleared specimens. Olympus’ line of silicone oil immersion objective offers excellent performance when observing these specimens. In this application note, we discuss two examples of high-resolution observation of deep tissues within cleared specimens using a FLUOVIEW® series confocal laser scanning microscope and silicone oil immersion objectives.
*Hama, Hiroshi, Hiroshi Kurokawa, Hiroyuki Kawano, Ryoko Ando, Tomomi Shimogori, Hisayori Noda, Kiyoko Fukami, Asako Sakaue-Sawano, and Atsushi Miyawaki. "Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain." Nature neuroscience 14, no. 11 (2011): 1481–1488.
Deep tissue observation of mouse brain sections that were rendered transparent with SCALEVIEW-A2
SCALEVIEW®-A2 is a product sold by Olympus that is used to render biological specimens transparent. SCALEVIEW-A2 eliminates light scattering without reducing the light absorption or fluorescence of formalin-fixed biospecimens. Immersing a mammalian brain specimen in SCALEVIEW-A2 solution causes it to become transparent. Without slicing brain specimens, structures labeled with fluorescent proteins, from the surface to deep in the tissue, can be observed in detail.
We imaged mouse brain sections that were rendered transparent with SCALEVIEW-A2 using a FLUOVIEW series confocal laser scanning microscope. Images were taken with a 60X oil immersion objective lens and compared to images taken with a 60X silicone oil immersion objective, the UPLSAPO60XS2.
| X-Z | X-Y | ||
| UPLASAPO60XO (NA: 1.35, W.D.: 0.15mm) | |||
| Z=5μm | Z=35μm, Scale bar: 5μm | ||
| |||
| Z=5μm | Z=35μm | ||
Comparison of deep tissue images taken of mouse cerebral neocortex sections rendered transparent and photographed with a 60X oil immersion objective lens and with a 60X silicone oil immersion objective. (Fluorescent antibody staining: VGluT1 (Green)/ VGluT2 (Red)/ MAP2 (Blue))
XY images from a mouse neocortex were taken with conventional oil immersion objectives and silicone oil immersion objectives. While bright fluorescence images were obtained with both sets of objectives, XY images at a depth of 35 μm were clearly brighter and had higher resolution when imaged with the silicone oil immersion objective.
This difference in brightness between the images taken at 35 μm is attributable to the oil immersion lens’ spherical aberration, which is caused by the difference in the refractive index between the type of oil used in the objective lens (ne ≒1.52) and the transparency-rendering solution, SCALEVIEW-A2 (ne ≒1.38).
The silicone oil immersion objectives perform better because the refractive index of silicone oil (ne ≒1.40) is closer to the refractive index of SCALEVIEW-A2, reducing the spherical aberration caused by mismatched refractive indices. This enables the user to capture brighter images.
In addition, silicone oil immersion objectives have a higher numerical aperture (NA) than water immersion objective lenses, so researchers can obtain higher resolution images deeper inside cleared tissues compared to what can be obtained using a water immersion objective lens.
The higher NA and reduced refractive index mismatch enable silicone oil immersion objectives to achieve excellent performance when conducting deep tissue observation on specimens rendered transparent with SCALEVIEW-A2.
Imaging system
Microscope: Olympus FV1200 confocal laser scanning microscope
Objective: UPLSAPO60XS2 (60X, NA: 1.30, W.D.: 0.3 mm)
Image data courtesy of
Motokazu Uchigashima, M.D., Ph.D., Masahiko Watanabe, M.D., Ph.D.
Department of Anatomy, Hokkaido University Graduate School of Medicine
Using silicone oil immersion objectives for deep tissue observation of a whole placenta rendered transparent with ScaleU2 clearing reagent
In an experiment published in the journal Development, researchers made deep tissue observations of specimens rendered transparent using ScaleU2 reagent with the 40X silicone oil immersion objective UPLSAPO40XS and a FLUOVIEW laser scanning microscope.**
In the experiment, a TG Mouse placenta (E10.5) expressing the fluorescence ubiquitination-based cell cycle indicator (Fucci) was cleared with ScaleU2 and fixed with agarose gel. Cells within the placenta were observed with 40X silicone oil immersion objectives and an inverted confocal laser scanning microscope. Use of ScaleU2 enabled researchers to make high-resolution observations of morphologically intact cells within the placenta without the need to prepare tissue sections. Additionally, since silicone oil immersion objectives are designed to have lower chromatic aberration, it is possible to precisely observe colocalization of Fucci (S/G2/M-Green, G1-Red) and the nucleus (DAPI-Blue).
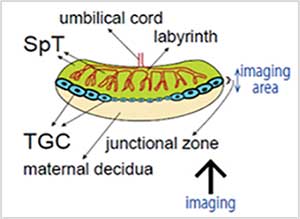
Figure 1: Observation of cells within a whole mouse placenta (E10.5)—outline drawing.
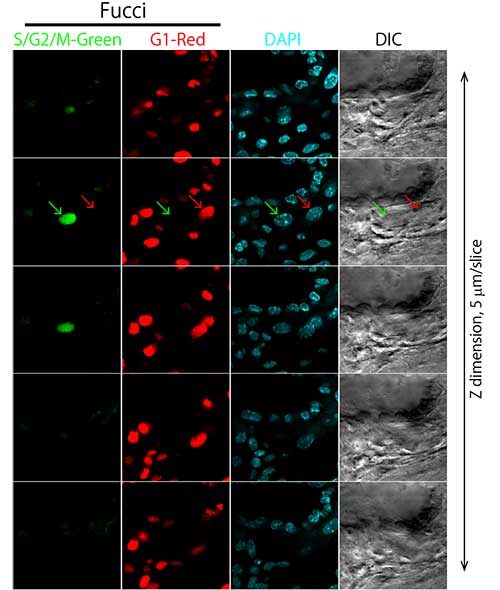
Figure 2: Observation of cells within a whole mouse placenta (E10.5)—sequential tomography.
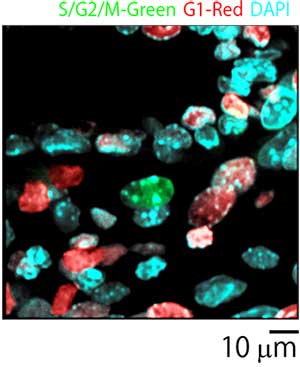
Figure 3: Merged image of placenta cells.
Imaging system
Microscope: Olympus FV1000 confocal laser scanning microscope
Objective: UPLSAPO40XS (40X, NA: 1.25, W.D.: 0.3 mm)
Diode laser: 405 nm, 473 nm, 559 nm
Image data courtesy of
Asako Sakaue-Sawano Ph.D., Atsushi Miyawaki M.D., Ph.D.
RIKEN Brain Science Institute Laboratory for Cell Function Dynamics
Reference
**Sakaue-Sawano, Asako, Tetsushi Hoshida, Masahiro Yo, Reiko Takahashi, Kenji Ohtawa, Takashi Arai, Eiki Takahashi, Shinichi Noda, Hiroyuki Miyoshi, and Atsushi Miyawaki. "Visualizing developmentally programmed endoreplication in mammals using ubiquitin oscillators." Development 140, no. 22 (2013): 4624–4632.
Conclusion
Olympus’ silicone oil immersion objectives offer both a high numerical aperture and long working distance. Since the refractive index of silicone oil (ne ≒1.40) is close to that of living tissue (ne ≒1.38), spherical aberration induced by a refractive-index mismatch is reduced for high-resolution imaging of thick living tissues. In addition, since silicone oil does not dry out, users don’t have to add additional oil over the course of their experiment, even across multiple days. The silicone immersion objectives are compatible with the IX-ZDC Z-drift compensator for long-term, stable, high-resolution 3D imaging that is consistently in focus.
Products Related to This Application
was successfully added to your bookmarks
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.