SpinSR10
Microscopes confocaux à disque rotatif et à très haute résolution
.jpg?rev=1162)
The Olympus SpinSR10 spinning disk confocal super resolution microscope has been integrated into IXplore SpinSR. The IXplore Systems are designed to provide solutions-based packages that suit your research application needs.
Not Available in Your Country
Sorry, this page is not
available in your country.
Caractéristiques
Higher Level of Super Resolution
Olympus Super Resolution
Olympus super resolution (OSR) technology is fast, easy to use, and can provide images from up to 100 microns deep within a cell in areas that are hard to access using other super resolution modes. Live cell super resolution images of internal cellular structures can be captured with 120 nm resolution from all kinds of samples using conventional fluorescent dyes. Processing on a single confocal image achieves super resolution imaging with minimum data volume as well as high speed.
Reference: S. Hayashi, "Resolution Doubling Using Confocal Microscopy Via Analogy With Structured Illumination Microscopy." Jpn J Appl Phys. (2016).

Olympus' Dedicated Magnification Changer
The Olympus dedicated magnification changer delivers even illumination across the entire field of view. The changer’s telecentric optical system optimized for the IX83 inverted microscope maximizes the performance of the objectives during confocal and super resolution imaging while enabling seamless switching between confocal and super resolution.

Fast Super Resolution Imaging and a Wide Field of View
Instead of painstakingly scanning the entire field of view, the sensitive imaging sensor on the SpinSR10 captures snapshots of the entire sample area in one step for fast imaging, enabling researchers to observe high-speed biological phenomena. In widefield and confocal mode, the microscope's optical system has a field number (FN) of 18 to capture images with a larger field of view, while two cameras enable users to simultaneously acquire dual-color super resolution images.
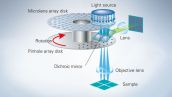
Spinning Disk Delivers Bright Live Cell Imaging
Each confocal pinhole on the disk has a microlens that enables you to image with lower laser power, reducing photobleaching and phototoxicity in your sample while enabling bright super resolution images.
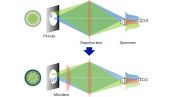
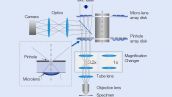
In regular confocal microscopes, image formation is a product of the illumination point spread function (PSF) and detection PSF. Looking at the image formation on the pinhole at position D from the optical axis, it’s the product of the illumination PSF and detection PSF, and we can see that information from position D/2 from the optical axis is transmitted but not resolved. To correct this, a microlens is fitted in the pinhole, and the individual focal points projected onto the pinhole are optically reassigned to the center, creating an ideal image and increasing the brightness and resolution. This process makes the resolution nearly equal to that of an ideal confocal microscope in which the pinhole has been reduced to an infinitesimal size.
Reference: T. Azuma and T. Kei, "Super-Resolution Spinning-Disk Confocal Microscopy Using Optical Photon Reassignment, " Opt. Express 23, 15003-15011 (2015).
Live Cell Super Resolution Imaging
The IXplore SpinSR10 system combines speed, reduced phototoxicity, and stability during time-lapse experiments to create 3D super resolution data that enables users to observe dynamic changes and phenomena within live cells.
Super Resolution for Living Samples
The spinning disk confocal optical system acquires live super resolution images at up to 200 frames per second.
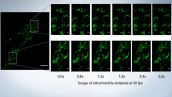
Image of mitochondria obtained at 30 fps
Mitochondria labeled by GFP. Acquired with 30 fps, able to see the individual mitochondria movements.
Image data courtesy of: Kumiko Hayashi, Ph.D., Graduate School of Engineering, Tohoku University
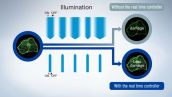
Real-Time Super Resolution*
High speed data processing algorithms enable the viewing of super resolution images in a live display window. This allows for real-time viewing of cellular activities compared to other computational super resolution techniques that require post processing before a super resolution image can be displayed.
EB3 proteins binding to the top of microtubles extending in HeLa live cells
EB3 proteins were GFP- labeled by means of transgenesis.
Image data courtesy of: Kaoru Kato, PhD, National Institute of Adovanced Industrial Science and Technology Biomedical Research Institute
Two-Color Simultaneous Imaging
The SpinSR10 system can use two cameras simultaneously to provide fast, two-color localization imaging.
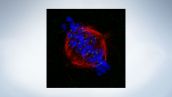
Mitotic spindle at metaphase cell*
HeLa cells derived from human cervical cancer were fixed and stained for α-tublin (microtubules, red) and Hec1 (kinetochores, green), respectively. DNA was stained with DAPI (chromosomes, blue).
Chromosomes interact with microtubules constituting mitotic spindle via kinetochores assembled on centromere region of chromosomes.
Image data courtesy of: Masanori Ikeda and Kozo Tanaka, Department of Molecular Oncology, Institute of Development, Aging and Cancer
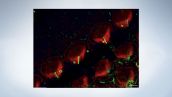
Stereocilia and kinocilia of inner hair cells in the organ of Corti (Actin:Orange, Tubulin:Green)
Image data courtesy of: Hatsuho Kanoh1, Toru Kamitani1,2, Hirofumi Sakaguchi2, Sachiko Tsukita1
1Graduate School of Frontier Biosciences and Graduate School of Medicine, Osaka University
2Department of Otolaryngology-Head and Neck Surgery, Kyoto Prefectural University of Medicine
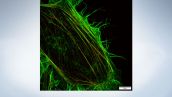
Stress fibers of Hela cell*
Antibody staining with Alexa Fluor 488 (green) for actin, Alexa Fluor 568 (red) for myosin heavy chain.
Image courtesy of: Keiju Kamijo,Ph.D. Division of Anatomy and Cell Biology, Faculty of Medicine, TOHOKU Medical and Pharmaceutical University
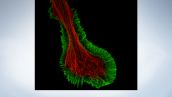
Fluorescent staining of microtubules (red: Alexa Fluor 594) and actin (green: Alexa Fluor 488) in growth cone of NG108 cells
Image courtesy of: Dr. Kaoru Katoh, Biomedical Research Institute, National Institute of Advanced Industrial Sciences and Technology (AIST)
Mitotic cultured epithelial cell. (Chromosome: Blue, Tubulin: Green, ZO1: Red):
Image courtesy of: Hatsuho Kanoh, Tomoki Yano, Sachiko Tsukita
Graduate School of Frontier Biosciences and Graduate School of Medicine, Osaka University
Keep Your Samples in Focus
During time-lapse imaging, minute changes in temperature, humidity, and other factors can cause your sample to go out of focus. TruFocus uses a low phototoxicity infrared laser to identify the sample plane and adjust the focus for clear time-lapse images. The continuous autofocus function works with glass and even plastic vessels.
Reduced Phototoxicity
The real time controller (U-RTCE) synchronizes the laser and camera with microsecond illumination accuracy to reduce photobleaching and phototoxicity, helping cells remain healthy during complex experiments.
See Inside Your Samples in Super Resolution
Observation at Depth
Users can clearly observe small individual spines not only on the surface of the sample, but also up to 100 microns deep within the sample.
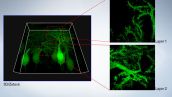
Purkinje cells labeled with GFP
XYZ image with confocal and super resolution image in different Z positions. Super resolution images are projected by Z (10 slices). 3D displayed by FV31S-DT.
Image data courtesy of: Yukari Takeo, Michisuke Yuzaki, PhD.
Department of Physiology, School of Medicine, Keio University
Image Three-Dimensional Structures
Obtain detailed three-dimensional super resolution image data during time-lapse imaging.
3D time-lapse of neuron
Time-lapse image of mouse primary neuron labeled with EGFP after co-culture with astrocyte for 2 weeks.
Easy to see the difference between immature spine (yellow arrow) and mature spine (blue arrow), and detect the morphological change in time. 3D was acquired with exposure time 500 ms/frame, 0.15 um Z step for 41 slices. Images were acquired every 2 minutes for 1 hour. 3D displayed by FV31S-DT.
Image data courtesy of: Yuji Ikegaya, PhD
Laboratory of Chemical Pharmacology, Graduate School of Pharmaceutical Sciences, The University of Tokyo
Optical Sectioning
Based on a confocal optical system, Olympus super resolution technology enables optical sectioning to acquire clear super resolution images with reduced background.

Mitotic epithelial cell (Chromosome: Blue, Tubulin: Green, ZO1: Red)
Image data courtesy of: Hatsuho Kanoh, Tomoki Yano, Sachiko Tsukita
Graduate School of Frontier Biosciences and Graduate School of Medicine, Osaka University
Improved Z Resolution
Olympus silicone immersion objectives are designed for deep tissue observation. Observation depth is negatively impacted by spherical aberration caused by refractive index mismatch. The refractive index of silicone oil (ne=1.40) is close to that of living cells or cultured tissue slices (ne=1.38), enabling super resolution imaging of internal cellular structures at tens of micrometers in depth with minimal spherical aberration.

In deep tissue observation, image quality depends on keeping the refractive index of the sample and immersion medium as close to each other as possible. When working with a silicone oil immersion objective, the difference between the refractive index of the samples and silicone oil is minimal. This objective achieves brighter fluorescence images with higher resolution for deep tissue observation.
Reduce Spherical Aberration
The remote correction collar unit is used to adjust the lens position within the objective to correct for spherical aberration caused by refractive index mismatch, resulting in dramatically improved signal, resolution, and contrast. The IX3-RCC unit works with any Olympus UIS2 objective that has a correction collar.

Sharp Super Resolution Images
Olympus' TruSight deconvolution works with super resolution images to create clear, sharp 3D images.

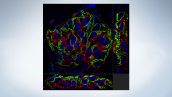
A Flexible System that Helps Simplify Your Research
Olympus cellSens image analysis software supports the complex experiments conducted with the IXplore SpinSR10 system. The software's efficient workflows enable users to effectively manage their data and perform advanced analysis that helps unlock new insights. The system integrates easily into existing protocols without necessitating major changes; labs can continue using their existing sample and labeling protocols.
One System, Three Imaging Modes
Researchers can use the imaging mode that most suits their sample. Users can switch between widefield, confocal, super resolution, and multicolor imaging with one click to locate areas of interest and then image fine structures.
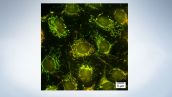
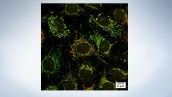

Easily Switch Observation Methods
The software makes it easy for you to change observation conditions. Switch between fluorescence channels, confocal, super resolution just by clicking a button.
Make Fine Adjustments
In super resolution imaging, the ability to make fine stage adjustments is critical. The highly accurate IX3-SSU ultrasonic stage is easy to use and can be controlled via software or the stage handle. The stage exhibits low thermal drift for reproducible multi-image acquisitions and stability during long term time-lapse experiments.


With new frame architecture and focus drive design, the IX3 system offers enhanced rigidity that reduces the impact of vibration and temperature. It maintains desired positions along the X, Y, and Z axes to facilitate reliable time-lapse and multipoint imaging.When combined with the Olympus IX3-SSU ultrasonic stage and Z drift compensator (TruFocus) , the system is perfectly suited for capturing high-precision, multipoint time-lapse images that are never out of focus or misaligned.

Manage Complex Experiments
The process manager makes it simple to acquire multicolor, Z-stack, and time-lapse images. The programmable graphic experiment manager (GEM) enables users to design more complex automation from a visual interface to support a wide variety of experimental imaging protocols and device triggering. Customize flexible experiment protocols that can be easily changed as needed anytime during the imaging process.
Powerful, Intuitive Image Analysis
Olympus cellSens imaging software enables various types of numerical data to be extracted from images obtained using the software's image analysis functions. Straight line distance, boundary length, or the area of a polygon can all be measured. The following additional advanced measurements are also possible:
Analyze Object Information
Analyze information about objects in your images, including the number of objects, area measurement, luminosity, and morphology.


Discriminate Spectrum Overlap
The colocalization function analyzes the fluorescent spectrum and discriminates between overlapping spectra.

Track Time-Lapse Imaging Data
During time-lapse imaging, the tracking function enables users to measure and analyze cell migration, division, and luminosity.


*Although it became one of the most important cell lines in medical research, it’s imperative that we recognize Henrietta Lacks’ contribution to science happened without her consent. This injustice, while leading to key discoveries in immunology, infectious disease, and cancer, also raised important conversations about privacy, ethics, and consent in medicine.
To learn more about the life of Henrietta Lacks and her contribution to modern medicine, click here.
http://henriettalacksfoundation.org/
Caractéristiques techniques
Super Resolution/Confocal Configuration | Confocal Configuration* | |||
| Laser Lines | 405 nm: 50 mW, 445 nm: 75mW, 488 nm: 100 mW, 514 nm: 40mW, 561 nm: 100 mW, 640 nm: 100 mW | |||
| Laser Combiner |
Main combiner: 405 nm, 488 nm, 561 nm, 640 nm + 1 line (445 nm or 514 nm)
Sub combiner: 445 nm, 514 nm 2x Interlock shutter available | |||
| Laser Light Control | Direct Modulation by U-RTCE, ultra-fast ON/OFF control and intensity modulation with individual laser lines, continuously variable (0 % - 100 %, 1 % increments) | |||
| Scanner | Yokogawa CSU-W1 | Disk Unit | SoRa 50 μm disk or 50 μm pinhole disk, maximally 2 disks selectable | Single 50 μm pinhole disk |
| Camera Port | 1 or 2 camera model** | 1 or 2 camera model | ||
| Super Resolution imaging | Acquisition Speed (max) | 5ms/f | - | |
| Optical Zoom | 3.2 X | - | ||
| Optical Resolution*** |
SoRa disk: 110 nm
50μm pinhole disk:120 nm | - | ||
| Field Number | 5.9 | |||
| Standard Resolution Imaging | Acquisition Speed (max) | 5ms/f | ||
| Optical Zoom | 1 X | |||
| Field Number | 18.8 | |||
| Dichromatic Mirror | 3 position (motorized slider) | |||
| Filter Wheel (emission) | 10 position (motorized wheel) | |||
| Imaging Sensor | HAMAMATSU ORCA Flash 4.0 V3 (CameraLink) | |||
| Microscope | Motorized Microscope | Inverted IX83 | ||
| Motorized Stage | IX3-SSU | |||
| Objectives for Super Resolution | UPLSAPO60XS2, UPLSAPO100XS, PLAPON60XOSC2, APON60XOTIRF, UAPON100XOTIRF | - | ||
| Super Resolution Adapter | Confocal/Super Resolution Lightpath Changer (Motorized) | |||
| Workstation | PC | OS: Windows 10 Professional 64-bit | ||
| Imaging Software | cellSens Dimension | Multi-Dimensional Acquisition and analyiss | ||
| Super Resolution Imaging Module | - | |||
* Confocal configuration is the system w/o super resolution function, able to upgrade to super resolution/confocal configuration
** Restrictions dependent on disk unit conbinations
***Typical experimental FWHM values with UPLSAPO100XS at 488 nm excitation. SoRa disk with 40 nm diameter beads and 50 μm pinhole disk with 100nm diameter beads.