Silicone Immersion Objectives Answer the Call for Higher Resolution
Refractive index mismatch, and resulting spherical aberration, has historically plagued researchers interested in long term, live-cell imaging at high resolution. The use of silicone oil objectives helps mitigate this mismatch as the refractive index of live cells (n = 1.38) is much closer to that of silicone oil (n = 1.4) than that of either traditional immersion oil (n = 1.52) or water (n = 1.33).
When used as an immersion medium, silicone oil greatly reduces spherical aberration, which is the inability of light rays to come to a single focal point in Z. As a result, brighter and higher resolution 3D images of live cells and living tissue can be acquired, especially at deeper sample depths. Furthermore, silicone immersion objectives yield morphological data that reflects actual sample morphology to a much greater degree, especially in the Z-dimension.
Silicone vs. Water or Oil
In the past, water immersion or glycerol immersion objectives have been primarily used for live-cell imaging. In the case of water immersion, its refractive index is low and it was not possible to develop high numerical aperture (NA) objective lenses at a given magnification. Resulting images therefore did not have sufficient light intensity for many applications. In contrast, the greater refractive index of silicone immersion objectives now enables the production of higher NA objectives. Silicone immersion objectives are indeed brighter than comparable water immersion objectives at all sample depths (Figure 1).
![]()
Figure 1: Brightness comparison of 60X objectives.
Oil immersion objectives are brightest at superficial depths. Silicone immersion objectives are brighter than water immersion objectives at all focus depths.
Silicone immersion oil demonstrates virtually no evaporation, even when used in 37 °C incubation chambers. This provides a significant advantage over water immersion objectives, as the researcher does not have to repeatedly open the incubator to administer the immersion medium. Not only does this require continual effort by the researcher, but it also exposes the sample to dramatic changes in temperature and CO2/O2, which can alter the health or physiology of the sample, and cause focus drift.
As glycerol is a highly hygroscopic substance, it easily changes its concentration depending on the humidity in its environment. This change in concentration means that glycerol’s refractive index can easily change.
Such variations provide a significant obstacle to researchers looking to perform longterm time-lapse imaging with glycerol immersion objectives. The refractive index of silicone remains constant despite changes in humidity, thus avoiding this issue.
One disadvantage of silicone immersion objectives is reduced viscosity relative to traditional oil. With lower surface tension and longer working distances, it may some times be challenging to initially position the oil in the correct location on top of the objective lens. This issue is not as problematic as it is for water immersion objectives and can be overcome through a number of simple strategies such as placing a bead of silicone oil on the coverslip instead of on the objective itself. Silicone oil objectives are also typically more expensive than traditional water or oil objectives.
Applications Suited for Silicone
Given the advantages of silicone immersion objectives, they are particularly useful in the areas of developmental biology, such as the macro and micro observation of embryos, zebrafish and other model organisms, as well as in regenerative biology for the investigation of the development and differentiation of embryonic stem and induced pluripotent stem (iPS) cells. In neurobiology they are useful for the high resolution imaging of brain slice and neural cultures.
Silicone immersion objectives are also ideal for certain superresolution applications due to their ability to produce an excellent point spread function at greater depths, where they easily outperform oil immersion objectives. They are also more effective than water immersion objectives for superresolution applications as they have a larger NA for comparable magnifications, reduced refractive index mismatch (Figure 2) and less evaporation.
Certain silicone objectives have special coatings with high transmission up to 1600 nm wavelengths, making them ideal for multiphoton imaging. Also, with a similar refractive index to some clearing agents, such as Olympus SCALEVIEW-A2 (n = 1.38), silicone objectives are well suited for advanced clearing techniques that produce less light scatter, enabling better resolution and deeper imaging.
![]()
Figure 2: Effects of refractive index mismatch on sample shape.
Matching the refractive index of a sample and immersion media is very important in the creation of accurate 3D images.
3D Live-Cell Imaging
Silicone immersion objectives enabled Kazuo Yamagata, Ph.D., associate professor from the Department of Genetic Engineering at Japan’s Kindai University, and his research team to obtain sharp 3D fluorescence images of proteins, DNA and other molecules in the zygote and of individual cells during embryonic development. High contrast 3D live-cell imaging during the in vitro development of a mouse embryo from the zygote to blastocyst stage over approximately four days was conducted using a silicone immersion objective (Olympus UPLSAPO60XS).
Yamagata and his researchers used a 60x silicone immersion objective for time-lapse 3D live-cell imaging. Yamagata’s team had previously used an oil lens and water immersion objective, the former standard for deep observation in live-cell imaging. By switching to a silicone immersion objective, the researchers were able to view fluorescently labeled methylated DNA (mCherry-MBD-NLS) within the nuclei from the surface to the inner region, from the one cell zygote to blastocyst stage (Figure 3).
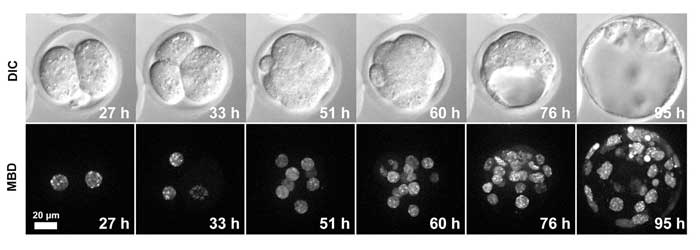
Figure 3: Live-cell imaging of a MethylRO embryo during pre-implantation development.
Changes in methylated DNA (mCherry-MBD-NLS) within the nuclei were observed over approximately 4 days. Images acquired using Olympus silicone immersion objective UPLSAPO60XS. Courtesy of Dr. Kazuo Yamagata, Associate Professor, Department of Genetic Engineering, Faculty of Biology-Oriented Science and Technology, Kinki University
Yamagata’s study shows that the use of silicone immersion objectives is essential for the realization of deep, high contrast 3D live-cell imaging over long periods of time. Yamagata and his team were able to obtain images that were constantly in focus and unaffected by temperature changes during long term observation.
Researchers Motokazu Uchigashima, M.D., Ph.D., and Masahiko Watanabe, M.D., Ph.D., from the Department of Anatomy at Japan’s Hokkaido University Graduate School of Medicine, obtained images of mouse brain sections with a 60x oil immersion objective and compared them with images taken with a 60x silicone immersion objective (Olympus UPLSAPO60XS2).
| X-Z | X-Y | ||
| UPLASAPO60XO (NA: 1.35, W.D.: 0.15mm) | |||
| Z=5μm | Z=35μm, Scale bar: 50μm | ||
| |||
| Z=5μm | Z=35μm | ||
Figure 4: Comparison of deep tissue images taken of mouse cerebral neocortex sections.
Comparison of deep tissue images taken of mouse cerebral neocortex sections rendered transparent and photographed with a 60x oil immersion objective lens and with a 60x silicone immersion objective lens. Courtesy of Motokazu Uchigashima, M.D., Ph.D., Masahiko Watanabe, M.D., Ph.D., Department of Anatomy, Hokkaido University Graduate School of Medicine.
X-Y images from a mouse neocortex were taken with conventional oil immersion objectives and silicone immersion objectives. While bright fluorescence images were obtained with both sets of objectives, X-Y images at a depth of 35 μm were clearly brighter and had higher resolution when imaged with the silicone immersion objective lens (Figure 4).This difference in brightness between the images taken at 35 μm is attributable to the oil immersion objective’s spherical aberration, which is caused by the difference in the refractive index between the type of oil used in the objective lens (1.52) and the transparency rendering solution (1.38). The higher NA and reduced refractive index mismatch of the silicone immersion objective used here enabled excellent performance while conducting deeptissue observation on specimens rendered transparent.
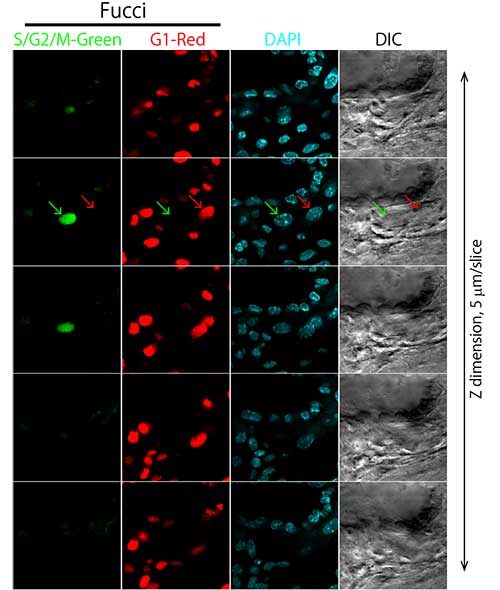
Figure 5: Observation of cells within a whole mouse placenta (sequential tomography).
Courtesy of Asako Sakaue-Sawano Ph.D., Atsushi Miyawaki M.D., Ph.D., RIKEN Brain Science Institute Laboratory for Cell Function Dynamics.
In a study undertaken by Asako Sakaue-Sawano, Ph.D., and Atsushi Miyawaki, M.D., Ph.D., of Japan’s RIKEN Brain Science Institute’s Laboratory for Cell Function Dynamics, researchers made deep tissue observations of whole mouse placenta specimens using a 40x silicone immersion objective (Olympus UPLSAPO40XS).
Lower Chromatic Variation
Cells within the placenta were observed with silicone immersion objectives and an inverted confocal laser scanning microscope. Researchers made high-resolution observations of morphologically intact cells within the placenta without the need to prepare tissue sections. Additionally, since silicone immersion objectives are designed to have lower chromatic aberration, it was possible to precisely observe colocalization of ubiquitination-based cell cycle indicators (Fucci) (S/G2/M-Green, G1-Red) and the nucleus (DAPI-Blue) (Figures 5 and 6).
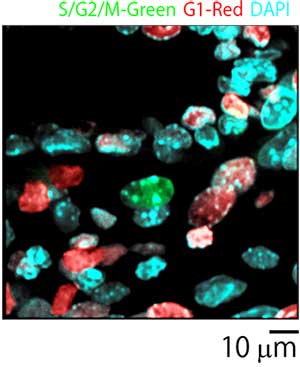
Figure 6: Merged image of placenta cells.
Courtesy of Asako Sakaue-Sawano Ph.D., Atsushi Miyawaki M.D., Ph.D., RIKEN Brain Science Institute Laboratory for Cell Function Dynamics.
The Optical Technology Group of ERATO Higashiyama Live-Holonics Project at Japan’s Nagoya University, a research group led by Daisuke Kurihara, Ph.D., recently succeeded in live-cell imaging of the plant zygote embryo genesis process. Within this study, long-term live-cell imaging of plant zygote division and growth was made possible by the development of a special medium and a new microdevice, the use of which included a silicone immersion objective (Olympus UPLSAPO30XS) (Figure 7). The process of embryogenesis was stably observed in real time for 67 hours from the early embryo (4-cell stage) to the late embryo.
![]()
Figure 7: Schematic illustration of microdevice imaging with a silicone immersion objective.
Multipoint time-lapse imaging was acquired using a motorized XY stage.
The world’s first long term real-time observation of embryogenesis was made possible by combining a newly developed ovule culture system and a microscope capable of high sensitivity imaging of the embryo, which is inside the ovule and covered by multiple layers of cells. The microscope system chosen for this study was the Olympus IX83 fully motorized inverted microscope with a 30x silicone immersion objective (Olympus UPLSAPO30XS). This objective has a high numerical aperture of 1.05 and a long working distance of 0.8 mm, which is required for deep, high definition imaging while retaining a wide field of view.
Summary
Silicone immersion objectives offer both a high NA and long working distance, enabling stable, long-term, high-resolution imaging that is unaffected by changes in temperature. With considerable improvements in spherical aberration over traditional lenses, silicone immersion objectives are ideal for various 3D, live-cell and deep tissue imaging applications done in the areas of developmental and regenerative biology, neurobiology, microbiology, and various other fields. By integrating silicone immersion objectives into their microscope systems, researchers can build a complete live-cell imaging system optimized for clarity, resolution and reproducibility.
*This article was originally published on Photonics Spectra.
Author
Andrew Samuelsson
Scientific Solutions Division
OLYMPUS CORPORATION OF THE AMERICAS
Sorry, this page is not
available in your country.