Within the structure of the cell, actin serves three distinct functions. Most prominently, it forms the most dynamic of the three subclasses of the cytoskeleton, which gives mechanical support to cells, and hardwires the cytoplasm with the surroundings to support signal transduction. Secondly, actin allows cell motility, the ability to move spontaneously and independently, in either single-celled or multicellular organisms. Thirdly, in muscle cells as well as non-muscle cells, actin helps generate force to support muscle contraction, vesicle movement, and other transport processes. In the digital video presented above, normal Gray fox lung fibroblast cells (FoLu line) are expressing a fusion of dimeric Eos (dEos) fluorescent protein to human beta-actin.
Video 1 - Run Time: 10 Seconds
Video 2 - Run Time: 08 Seconds
Video 3 - Run Time: 08 Seconds
Video 4 - Run Time: 14 Seconds
Video 5 - Run Time: 08 Seconds
A focal contact is a highly specialized type of attachment between actin filaments and the extracellular matrix, and it allows cells to pull on the substratum to which they are bound. Focal contacts are especially easy to see in cultured fibroblasts (cells that give rise to connective tissue), because they create spots where the normal 50 nanometer gap between the bottom of the cell and the substratum is reduced to only 10-15 nanometers. At these sites, stress fibers—consisting of contractile bundles of actin and myosin II filaments—terminate at the plasma membrane, where clusters of transmembrane adhesion proteins called integrins are located. In the digital video presented above, normal Gray fox lung fibroblast cells (FoLu line) are expressing a fusion of dimeric Eos (dEos) fluorescentss protein to human beta-actin.
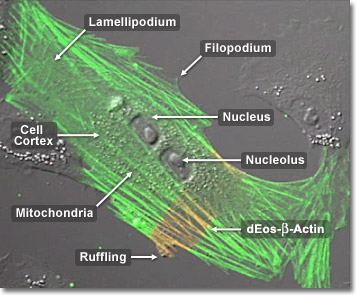
Actin can be linked to the plasma membrane in a number of ways. The most basic mode of attachment is by direct binding to an integral membrane protein. However, the more common interaction is indirect, and many proteins are known to act as linkers, or adapters, between actin filaments and membrane proteins. Both alpha-actinin and filamin can act in this way, as well as many other specific proteins in red blood cells, cell junctions and in actin-rich extensions of the cell surface. Each membrane linker is adapted to function in its particular location but all must have at least two distinct domains, one that binds to actin and another that binds to an integral membrane protein. In the digital video presented above, normal Gray fox lung fibroblast cells (FoLu line) are expressing a fusion of dimeric Eos (dEos) fluorescent protein to human beta-actin.