Combination Methods with Differential Interference Contrast (DIC)
To minimize the effects of photobleaching, fluorescence microscopy can be combined with other techniques that are non-destructive to the fluorochrome, such as differential interference contrast (DIC), Hoffman modulation contrast (HMC), transmitted darkfield illumination, and phase contrast.
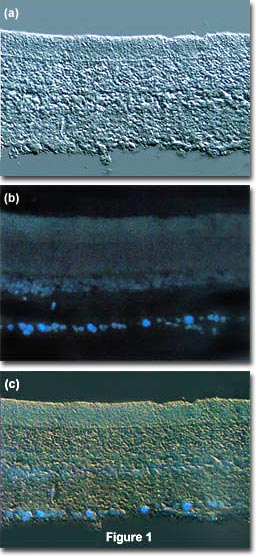
The idea is to locate a specific area of interest in a specimen using the non-destructive contrast enhancing technique then, without relocating the specimen, switch the microscope to fluorescence mode. The results of a typical experiment of this type are illustrated in Figure 1. Figure 1(a) illustrates a rat retina optic ganglion tissue thin section imaged using differential interference contrast. The photomicrograph in Figure 1(b) shows the same viewfield, but this time imaged using fluorescence illumination with cells stained by the fluorochrome fast blue, a diazonium salt that specifically stains phospholipids in the myelin sheath. Figure 1(c) illustrates the two techniques used in combination to produce a beautiful photomicrograph of fluorescent-stained optic ganglion lipid tissue superimposed on a differential interference contrast image of the retina. This image was recorded with a specialized fluorite objective designed to permit simultaneous observation of both fluorescence and differential interference contrast with the same objective.
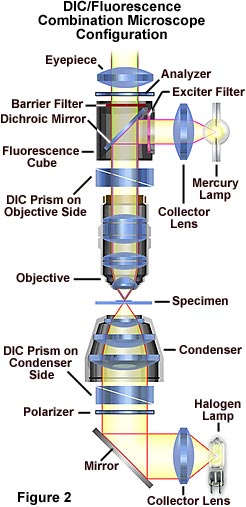
The microscope configuration typically utilized to simultaneously image specimens using both differential interference contrast (DIC) and fluorescence illumination is illustrated in Figure 2. DIC is conducted using transmitted light provided by a tungsten-halogen lamp positioned in a lamphouse attached to the microscope base. Light passing through the field diaphragm is reflected by a mirror into the substage condenser and through a Wollaston prism located at the front focal plane of the condenser.
Fluorescence Combination Microscopy
Explore combinations of fluorescence microscopy with additional contrast-enhancing techniques using both phase contrast and differential interference contrast (DIC) methods.
Light diffracted by the specimen is first passed through a second Wollaston prism positioned near the objective rear focal plane before interfering at the intermediate image plane with light passing through the specimen undiffracted. Simultaneously, ultraviolet light emitted by a mercury burner is passed through an exciter filter, then reflected by a dichroic mirror onto the specimen from above. Secondary fluorescence emitted by the chromophore attached to the stained specimen is captured by the objective and passed through the barrier filter and into the eyepieces and/or phototube. This configuration can be used to image specimens using the techniques (fluorescence and differential interference contrast) individually or in combination.
Sorry, this page is not
available in your country.