An epitope (also known as an antigenic determinant) is a biological structure or sequence, such as a protein or carbohydrate, which is recognized by an antibody as an antigen. Recognition of the antigen occurs when an appropriate structure is formed in an area of a protein or polysaccharide in which amino acids or sugars are arranged linearly. Most proteins usually have several kinds of epitopes. The antibody offers an important technique (termed an immunoassay) for identifying specific cellular components (proteins, lipids, carbohydrates, etc.) to track the function, distribution, and modification of the protein of interest within living and fixed cells.
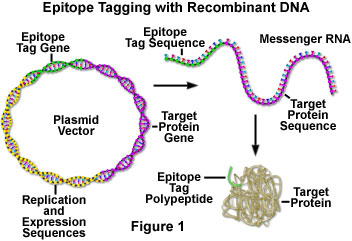
The antibody is usually obtained by refining the protein of interest and injecting it in a rabbit or mouse. However, this takes a significant period of time and there are many cases in which antibody creation fails (absence of epitope). However, recent advances in DNA recombination technology have made it possible to create a fused protein in which the protein of interest is bonded with a small fragment of protein (peptide) working as the epitope. The proteins tagged with an epitope can then be detected by using a specific antibody for the epitope.
The use of epitope tagging is similar to the approach for constructing recombinants of the green fluorescent proteins (GFPs). The gene from the target protein is inserted into the epitope tag vector and the target protein with its tag is expressed in cells by transfection of the vector. The protein is distributed inside the cell according to the original properties of the protein. When using GFPs, the target protein can be observed easily because the GFP tag emits fluorescence; however, when using the epitope tag, the target proteins can be detected only by immunological procedures because the epitope tag is not functional in cells.
Advantages of Epitope Tagging
- Recombination methods offer a very short time requirement compared to ordinary antibody preparation, which requires more than one month to immunize the test animal.
- Using a single epitope tag, a variety of proteins in specimens can be detected by the same tag-specific antibody.
- Recombination tagging can be done at a lower cost than antibody preparation.
- The epitope tag is composed of 3 to 14 amino acids and does not disrupt normal protein functions.
- Epitope tagging can be used to distinguish between two proteins with similar antigenicity.
- The technique can be used to detect proteins that are very difficult to isolate and purify.
The following table shows the amino acid arrangements that are often used as epitope tags:
Common Epitope Tags
| Name | Amino Acid Quantity | Amino Acid Sequence |
|---|---|---|
| HA | 9 | YPYDVPDYA |
| FLAG | 8 | DYKDDDDK |
| C-MYC | 10 | EQKLISEEDL |
Table 1
Detection
Both the direct and indirect methods can be used like the fluorescent antibody technique. With the direct method, the antibody for the tag is labeled with a fluorescent dye, while with the indirect method, the antibody against the tag is applied as the primary antibody and detection is performed on the secondary antibody that is labeled with a fluorescent dye.
For more information on epitope tagging, please refer to the Experiment Manual in the Roche website.
One-Letter Amino Acid Codes
| A - Alanine | C - Cysteine | D - Aspartate | E - Glutamine |
| F - Phenylalanine | G - Glycine | H - Histidine | I - Isoleucine |
| K - Lysine | L - Leucine | M - Methionine | N - Asparagine |
| P - Proline | Q - Glutamine | R - Arginine | S - Serine |
| T - Threonine | V - Valine | W - Tryptophan | Y - Tyrosine |
Table 2
Internet Resources
- Epitope Tagging Basic Laboratory Methods - Available as a series of portable document files (PDFs) from Roche Applied Science, the epitope tagging manual contains an introduction and sections about experimental procedures. Critical factors for success in the process are reviewed and tips for getting started are offered.