FV10i-LIV Microscopios de barrido láser
Productos discontinuados

This product has been discontinued, check out our current product
The unique FLUOVIEW FV10i-LIV is a fully automated confocal laser-scanning microscope. The completely re-engineered design of this microscope, integrated into a self-contained package with a variety of functions, enables even inexperienced and first-time users to perform easily and efficient high-quality confocal imaging. Olympus has made no compromises in ergonomics and image quality, using high-quality optical components and smart and easy software.
Not Available in Your Country
Sorry, this page is not
available in your country.
Características
Unique Self-contained Confocal Laser Scanning Unit
A Unique, Easy-to-use, Self-contained Confocal Laser Scanning Microscope with a Small Footprint
The FV10i features a unique self-contained design. Simplified and optimized, key components, including an incubator and laser combiner, have been integrated into a self-contained package with no compromises. The system, which is easier to set up and use, features the same functionality as a high-end confocal laser scanning microscope while adding vibration isolation and a light tight cover. As a result, the FV10i can be operated by new and experienced users alike and, with a compact footprint, can be installed in any laboratory without the need for a dedicated dark room.

| 1.Dark Room Free | The Microscope body and light tight cover are integrally combined. The FV10i can be used with ease in a laboratory, unlike conventional confocal laser scanning microscopes which may require a dark room. |
|---|---|
| 2.Scanning Unit | The system is equipped with a detector which automatically sets conditions in accordance with fluorescence dye on a scanning unit. Imaging can be performed in the conditions that are most suited for each fluorescence dye. |
| 3.Microscope Function | The FV10i's excellent optical and mechanical modules are totally integrated. The FV10i can capture images from 10× to 600× magnification with 10×, 60× objectives and optical zoom. |
| 4.Vibration Isolation Function | Equipped with built-in vibration insulators. A vibration isolation table is not required and so can be installed directly on an experimental tabletop. |
| 5.Laser Combiner | Equipped with four diode laser units, each unit utilizes a compact diode laser for longer life and power-saving compared to traditional confocal systems. |
High-definition Images using Advanced Optics
Equipped with 4 Diode Lasers

The system is equipped with four (405/473/559/635nm) lasers. Multi-stained specimens can be imaged with up to four fluorescence dyes. Maintenance-free and power-saving diode lasers with longer operating lives are employed in all the laser units, and operate with low noise levels.
Detector Utilizes a Newly Developed Spectrum Method

The detecting mechanism has two fluorescence channels, and one phase contrast channel. The fluorescent channels use a newly developed spectrum method comprising grating, beam splitter, and slit. In addition, they are equipped with the variable barrier filter function where the most suitable wavelength width is set automatically in accordance with the characteristics of the fluorescence dye.
Two Sequential Modes
The FV10i is equipped with two sequential modes. Images can be acquired through line sequences without crosstalk in imaging with two fluorescence dyes, and with three or four dyes in frame sequences with the virtual channel function.
Objectives of 10× and 60× are System-mounted

The system is equipped with objectives of 10× and 60×. Zoom magnification can be changed continually from 10× to 600×. The most suitable imaging area can be set depending on the size of the specimen.
Built-in Incubator Ideal for Time-lapse Imaging
Simplified Built-in Incubator

The system has a simplified built-in incubator, allowing easy time-lapse imaging of live cells without losing valuable time in setting up equipment. The environment in the culture chamber is maintained at a temperature of - 37 degrees Celsius, humidity of - 90 %, and CO2 concentration of - 5 %* . Time-lapse imaging up to a maximum of three days is supported.
* To maintain 5% of CO2 in dish, injection of 6 % CO2 with 150 ml/min is recommended.
A Dedicated Culture Pod is Provided

The system is provided with a dedicated culture pod for dia. 35 mm glass bottom dishes. Recirculation of the culture media and addition of a medicinal solution during time-lapse is possible. In addition, the culture pod system can be autoclaved for sterilization.
Stable Time-lapse Imaging
Not only the incubator but also the surrounding air space is maintained at 37 degrees
Celsius*. Long-term time-lapse imaging is, therefore, possible while maintaining cell activity.
*Fluctuation of ambient temperature may affect focusing stability.
Water is Automatically Supplied to the Water-immersion Objective

The newly developed automatic water dispensing system enables the FV10i to supply water to the top of the water-immersion objective. Long term time-lapse imaging can continue without concern for immersion media levels. Water is supplied automatically when the objective is moved into the observation position.
Detection of Cover Glass Thickness and Automatic Adjustment of the Correction Collar

The system is equipped with the capability to detect the thickness of the cover glass, allowing it to adjust the correction collar automatically, when using the water-immersion objective. This assures imaging is performed each time with optimal conditions.
Supports Multi-area Time-lapse Imaging
The system is equipped with a motorized stage, and imaging is possible through multi-area time-lapse. Ten point locations can be assigned within a single dish (well). For example, in the case of a dia. 35mm glass bottom dish, three dishes can be mounted, allowing a maximum of up to 30 locations to be captured.
Start Capturing Images from the First Day
Stress-free Operation for Every User
Only two manual steps are required of the user: placing of the specimen on the stage and closing the cover. After that, the sophisticated user interface offers clear and efficient operation of the microscope. The selection of the imaging point, for example, can be performed easily using the newly designed image mapping menu without need for special experience or expertise. Furthermore, using advanced functionality that is only found in Olympus products, automatic focus and intensity adjustment allow the imaging conditions to be set up according to the type of specimen and observation mode required. In addition, the system is equipped with a navigation function that identifies the operational step of the imaging procedure and guides the operator to the next appropriate operation. The FV10i confocal laser scanning microscope, therefore, provides a stress-free and comfortable operating environment even for first-time users.
Setting
Place a specimen, and select a fluorescence dye. The FV10i automatically selects the most suitable imaging conditions based on the fluorescence dye selection.
Image Mapping Menu
Just click the <Start> button, and a map image of the specimen is created automatically. Users can then easily identify the desired image acquisition point.
Image Capturing
Through the sophisticated operating software, the image capture area or zoom magnification can be set quickly. Image capture is then completed at the click of a button.
Capture a Map Image Easily for Navigation
When the Specimen Has Been Loaded, Clicking <Start> Begins the Mapping Process
Following the loading of the specimen, a map image can be acquired by simply clicking the <Start> button in the "Acquire Map Image" window. With this bird's-eye view of the specimen, the user can quickly and easily select the imaging area to be captured.
Create Map Area
The map image provides the user with an overview of the possible image capture area. The selectable map areas are automatically displayed in a diagram according to the type of specimen holder that has been used, such as a 35 mm diameter dish or glass slide. An area can be selected and map image acquired by clicking on the desired scan area in the diagram. This will display an overview image of the area on the “Map Image” screen. Selecting an alternative area requires just a single click operation.
Fluorescence Dye Selection
A separate map image can be displayed for each fluorescence dye. These images can be displayed individually or be overlaid with each other.
Scanning Setting
One of two scanning orders can be selected depending on the experimental requirements.
Automatic
A map image is automatically acquired from the center outward in a spiral pattern, allowing even a first-time user to easily identify the confocal view area.
Manual
The desired map image areas can be selected directly up to a maximum 9 × 9 area. Manual selection is more efficient than automatic selection as the ROI (Region of Interest) can be narrowed down in advance.
A Map Image of the Specimen Can be Automatically Created

A map image can be created by automatically detecting which type of specimen holder is used. High speed with low resolution or low speed with high resolution image mapping options is available.
Easy Guided Operation for First-time Users
Sophisticated Menus Allow Easy Navigation of Imaging Area
The desired imaging region can be selected using the map image and live image screens utilizing the zooming function of the intuitive user interface. The user friendly navigation functions allow even a first-time user to capture images with ease.
| Observation Mode Selection |
Five types of observation modes can be selected including time-lapse, Z-stack, and multi-area
|
|---|---|
| Multi-area Setting | Register the areas for imaging in multi-area mode. Appropriate imaging conditions can be set for each area. |
| Map Image | The image acquired in [Acquire Map Image] is displayed. Specific regions can be selected for closer examination. |
| Control Screen | Imaging conditions can be set in detail with operation of various controllers. Main settings include:
|
| Live Image | The selected point from the map image screen is displayed. The imaging area is determined through the framing and zooming functions. It is possible here to switch between the displays for each type of fluorescence dye. |
The System is Equipped with a User Friendly Navigation Function
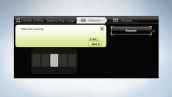
Clicking the <Navigation> button shows the operational procedure and highlights the operational button. Just follow the navigational guidance to easily complete your imaging.
Stitching Function

Wide-angle high-resolution imaging can be obtained by acquiring the adjacent regions. A map image of the entire glass slide can also be created.
Software ZDC Function (FV10i-LIV Only)

Z-drift compensation function (software ZDC) reduces Z-drift by temperature-shift in time-lapse imaging.
Dedicated Analysis Software Exclusive to FLUOVIEW
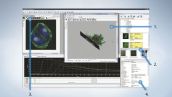
Dedicated Olympus software is provided as part of the standard specifications, bringing editing and analysis capabilities to images taken by FV10i.
| 3D Display Function(No.1) | The FV10i supports the Alpha Blend method and Maximum Intensity Projection method for the 3D display function. Also, the system is equipped with various display functions which allow a user to freely change the angle of 3D images and section the image at any spot. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Easy Image Searching(No.2) | Thumbnail list is possible on the main screen allowing a user to easily search for previous image data. | ||||||||
| 2D Analysis Tool(No.3) |
| ||||||||
| Data Manager(No.4) | The data manager displays thumbnails and various file information with clarity. |
File Input/Output
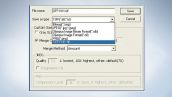
OIF (Olympus Image Format) is employed to store various parameter settings and images together. This software supports a wide range of well-used formats with high interchangeability including TIFF, BMP and JPEG.
Feature-rich Functionality for Stress-free Imaging
Equipped with various Specimen Holders

The system is equipped with specimen holders, usable for a dia. 35 mm glass bottom dishes, glass slides, cover glass chambers (8 wells type), and well slides (8 wells type). Specimens can be observed without the risk of contamination using the closed contamination-free plastic cover of a dia. 35 mm glass bottom dish.
Capturing Adjacent Images in Wide field
Imaging of adjacent images is possible to create big mosaic images. These can be captured in high-definition and wide field of view.
HDD Recording for Storing Large Volumes of Data
The microscope comes equipped with a HDD (hard-disk drive) recording function. The images captured are stored automatically in the HDD. Large volumes of data, such as those obtained from long-term time-lapse imaging can be stored. During imaging, editing/analysis of previously taken images is also possible. An external HDD connected to a network can be specified as the destination, and the saved images can be viewed on a remote PC while performing separate imaging.
Especificaciones
| Laser Unit > Qualified IR Pulsed Laser with Negative Chirp for Multiphoton Excitation | - | |
|---|---|---|
| Laser Unit > Automatic Introduction Optic |
Built-in
| |
| Laser Unit > Optional Visible Light Laser for Stimulation | - | |
| Laser Unit > LD Laser for Visible Light > 405 nm |
| |
| Laser Unit > LD Laser for Visible Light > 440 nm |
| |
| Laser Unit > LD Laser for Visible Light > 473 nm |
| |
| Laser Unit > LD Laser for Visible Light > 559 nm |
| |
| Laser Unit > LD Laser for Visible Light > 635 nm |
| |
| Laser Unit > HeNe(G) Laser (543 nm, 1 mW) |
| |
| Scanning and Detection > Main Scanner > Standard Laser Ports |
| |
| Scanning and Detection > Main Scanner > Detector > Standard |
| |
| Scanning and Detection > Main Scanner > Detector > Cooled GaAsP-PMT 2 CH |
| |
| Scanning and Detection > Main Scanner > Detector > Optional 4 CH |
| |
| Scanning and Detection > Main Scanner > Photo Detection Method > Analog Integration |
| |
| Scanning and Detection > Main Scanner > Photo Detection Method > Hybrid Photon Counting |
| |
| Scanning and Detection > Main Scanner > VIS - UV - IR Excitation Dichromatic Mirror Turret |
| |
| Scanning and Detection > Main Scanner > Beamsplitter Turrets |
| |
| Scanning and Detection > Main Scanner > Wavelength Selection | Variable barrier fi lter mechanism for fl uorescence channel by diffraction grating and slit | |
| Scanning and Detection > Galvanometer Scanner (Normal Imaging) > Galvanometer Mirror Scanner (X, Y) |
| |
| Scanning and Detection > Galvanometer Scanner (Normal Imaging) > Scanning Modes > 2D | XY | |
| Scanning and Detection > Galvanometer Scanner (Normal Imaging) > Scanning Modes > 3D | XYT, XYZ | |
| Scanning and Detection > Galvanometer Scanner (Normal Imaging) > Scanning Modes > 4D | XYZT | |
| Scanning and Detection > Galvanometer Scanner (Normal Imaging) > Scanning Modes > 5D | - | |
| Scanning and Detection > Galvanometer Scanner (Normal Imaging) > Scanning Modes > Other |
Map image, one shot
| |
| Scanning and Detection > Galvanometer Scanner (Normal Imaging) > Scanning Speed |
| |
| Scanning and Detection > Galvanometer Scanner (Normal Imaging) > Pinhole |
Single motorized
| |
| Scanning and Detection > Galvanometer Scanner (Normal Imaging) > Scanning Zoom |
| |
| Scanning and Detection > Resonant Scanner (High-Speed Imaging) > Scanning Modes > 2D | - | |
| Scanning and Detection > Resonant Scanner (High-Speed Imaging) > Scanning Modes > 3D | - | |
| Scanning and Detection > Resonant Scanner (High-Speed Imaging) > Scanning Modes > 4D | - | |
| Scanning and Detection > Resonant Scanner (High-Speed Imaging) > Scanning Modes > 5D | - | |
| Scanning and Detection > Resonant Scanner (High-Speed Imaging) > Scanning Modes > other | - | |
| Scanning and Detection > Resonant Scanner (High-Speed Imaging) > Scanning Speed | - | |
| Scanning and Detection > Resonant Scanner (High-Speed Imaging) > Scanning Zoom | - | |
| Scanning and Detection > Field Number (NA) |
| |
| Scanning and Detection > Z-Drive |
| |
| Scanning and Detection > Transmitted Light Detector Unit | Phase Contrast: 1 channel | |
| Microscope > Frame |
| |
| Microscope > Frame > Inverted |
| |
| Microscope > Objectives and Focus > Objectives |
| |
| Microscope > Objectives and Focus > Auto Focus (AF) |
| |
| Microscope > Objectives and Focus > Water Supply |
| |
| Microscope > Objectives and Focus > Oil Supply |
| |
| Microscope > XY Stage > XY Driving Method |
| |
| Microscope > XY Stage > Specimen Holder |
| |
| Microscope > Incubator > Room Environment |
| |
| Microscope > Incubator > Heating Method |
| |
| System Control > Controller |
| |
| System Control > Power Supply Unit | - | |
| Optional Unit > SIM Scanner |
| |
| Optional Unit > TIRFM Unit |
| |
| Software > Image Acquisition | - | |
| Software > Programmable Scan Controller | - | |
| Software > 2D Image Display | - | |
| Software > 3D Visualization and Observation | - | |
| Software > Image Format | - | |
| Software > Spectral Unmixing | - | |
| Software > Statistical Processing | - | |
| Software > Optional Software | - | |
| Software > Image Acquisition Mode |
Map image, one shot, time-lapse (XYT), Z-stack (XYZ), Z-stack time-lapse (XYZT), multi area time-lapse (Multi Area XYT), multi area
| |
| Software > Map Image Acquisition | Automatic selection of map image of 3 x 3 – 35 x 7 fields according to 10 X objective lens (The maximum area varies in accordance to the specimen holder used), and manual selection of map acquisition area | |
| Software > Multi Area Time-Lapse |
Automatic multi area time-lapse by motorized XY stage
| |
| Software > Image Acquisition Area | Area appointment: All area, clipping square area (minimum area: 96 x 96 pixels) | |
| Software > Image Display |
| |
| Software > Cross Talk Reduction | Line sequential action (2 channel), or frame sequential action (3 channel and 4 channel) | |
| Software > Acquisition Image File Type |
| |
| Software > Image File Type Available for Viewing |
| |
| Software > Image Editing | LUT: pseudo color setting, contrast adjustment, Comment: inputting graphic, text, scale etc., image extraction, combination | |
| Software > 3D Image Construction | 3D display: AlphaBrend method, Maximum intensity projection method 3D animation display, free orientation of cross section display | |
| Software > IR Laser Controlling |
| |
| Dimensions, Weight and Power Consumption > Microscope with Scan Unit > Dimensions (mm) |
| |
| Dimensions, Weight and Power Consumption > Microscope with Scan Unit > Weight (kg) |
| |
| Dimensions, Weight and Power Consumption > Microscope with Scan Unit > Power Consumption |
| |
| Dimensions, Weight and Power Consumption > Fluorescence Illumination Unit > Dimensions (mm) |
| |
| Dimensions, Weight and Power Consumption > Fluorescence Illumination Unit > Weight (kg) |
| |
| Dimensions, Weight and Power Consumption > Fluorescence Illumination Unit > Power Consumption |
| |
| Dimensions, Weight and Power Consumption > Transmitted Light Detection Unit > Dimensions (mm) |
| |
| Dimensions, Weight and Power Consumption > Transmitted Light Detection Unit > Weight (kg) |
| |
| Dimensions, Weight and Power Consumption > Transmitted Light Detection Unit > Power Consumption |
| |
| Dimensions, Weight and Power Consumption > Microscope Control Unit > Dimensions (mm) |
| |
| Dimensions, Weight and Power Consumption > Microscope Control Unit > Weight (kg) |
| |
| Dimensions, Weight and Power Consumption > Microscope Control Unit > Power Consumption |
| |
| Dimensions, Weight and Power Consumption > FV Power Supply Unit > Dimensions (mm) |
| |
| Dimensions, Weight and Power Consumption > FV Power Supply Unit > Weight (kg) |
| |
| Dimensions, Weight and Power Consumption > FV Power Supply Unit > Power Consumption |
| |
| Dimensions, Weight and Power Consumption > Power Supply Unit for Laser Combiner > Dimensions (mm) |
| |
| Dimensions, Weight and Power Consumption > Power Supply Unit for Laser Combiner > Weight (kg) |
| |
| Dimensions, Weight and Power Consumption > Power Supply Unit for Laser Combiner > Power Consumption |
| |
| Dimensions, Weight and Power Consumption > Laser Combiner (with Ar Laser Heads) > Dimensions (mm) |
| |
| Dimensions, Weight and Power Consumption > Laser Combiner (with Ar Laser Heads) > Weight (kg) |
| |
| Dimensions, Weight and Power Consumption > Laser Combiner (with Ar Laser Heads) > Power Consumption |
| |
| Dimensions, Weight and Power Consumption > Laser Combiner (without Ar Laser Heads) > Dimensions (mm) |
| |
| Dimensions, Weight and Power Consumption > Laser Combiner (without Ar Laser Heads) > Weight (kg) |
| |
| Dimensions, Weight and Power Consumption > Laser Combiner (without Ar Laser Heads) > Power Consumption |
| |
| Dimensions, Weight and Power Consumption > LD559 Laser Power Supply > Dimensions (mm) |
| |
| Dimensions, Weight and Power Consumption > LD559 Laser Power Supply > Weight (kg) |
| |
| Dimensions, Weight and Power Consumption > LD559 Laser Power Supply > Power Consumption |
| |
| Dimensions, Weight and Power Consumption > Multi Ar Laser Power Supply > Dimensions (mm) |
| |
| Dimensions, Weight and Power Consumption > Multi Ar Laser Power Supply > Weight (kg) |
| |
| Dimensions, Weight and Power Consumption > Multi Ar Laser Power Supply > Power Consumption |
| |
| Dimensions, Weight and Power Consumption > HeNe(G) Laser Power Supply > Dimensions (mm) |
| |
| Dimensions, Weight and Power Consumption > HeNe(G) Laser Power Supply > Weight (kg) |
| |
| Dimensions, Weight and Power Consumption > HeNe(G) Laser Power Supply > Power Consumption |
| |
| Dimensions, Weight and Power Consumption > Operating Environment (Indoor Use) > Ambient Temperature | 18 - 28 ℃ (fluctuation ±2 ℃) | |
| Dimensions, Weight and Power Consumption > Operating Environment (Indoor Use) > Maximum Relative Humidity | 30 - 80% (sin condensación) |
Aplicaciones
Visualization of Physiological State of Stem Cells Using Cell Cycle Marker Fucci
|
Spheroids consisted of CD133-positive human gastric cancer cells with cell cycle marker Fucci were cultured and observed continuously over an extended period. The upper panels in the slide shows that spheroids maintained their stem cell state as they were cultured without serum, leading to a halt in the cell cycle. On the other hand, in the lower panels, spheroids have been cultured with serum, which is responsible for cell division. The images indicate that these spheroids are
shifting into a monolayer culture state (losing their stem cell-like state). The confocal laser scanning microscope FV10i enabled the observation of thick spheroids in three dimensions.
*The images were taken serially for 48 hours with the confocal laser scanning microscope FV10i. |
Image data courtesy of:
Shuya Yano, Toshiyoshi Fujiwara
Department of Gastroenterological Surgery Transplant and Surgical oncology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences.
Reference:
Yano S, Tazawa H, Hashimoto Y, Shirakawa Y, Kuroda S, Nishizaki M, Kishimoto H, Uno F, Nagasaka T, Urata Y, Kagawa S, Hoffman RM, Fujiwara T. A genetically engineered oncolytic adenovirus decoys and lethally traps quiescent cancer stem-like cells into S/G2/M-phases. Clin Cancer Res. December 1, 2013 19:6495-6505.
Activation and Cell Destruction of Stem Cells from Sleeping Cancer by Telomelysin (OBP-301)
|
The CD133-positive, stem-like human gastric cancer cells with the cell cycle marker Fucci which had been cultured as spheroids were treated with either telomelysin (OBP-301), cisplatin or radiation. The clumps of cancer cells treated with cisplatin or radiation remained the same size and the majority of the cells did not proceed the cell cycle beyond the G1 phase (seen in red). On the other hand, the clump of cancer cells treated with telomelysin showed a change in color from red to
yellow and green, and a gradual decrease in size. This indicates that telomelysin inhibits the expression of p53 and p21, both of which are responsible for arresting the cell cycle of stem-like cells. Telomelysin also increases the expression of E2F-1, which leads to the activation of the cell cycle by contraries.
*The images were taken serially for 8 days with the confocal laser scanning microscope FV10i. |
Image data courtesy of:
Shuya Yano, Toshiyoshi Fujiwara
Department of Gastroenterological Surgery Transplant and Surgical oncology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences.
Reference:
Yano S, Tazawa H, Hashimoto Y, Shirakawa Y, Kuroda S, Nishizaki M, Kishimoto H, Uno F, Nagasaka T, Urata Y, Kagawa S, Hoffman RM, Fujiwara T. A genetically engineered oncolytic adenovirus decoys and lethally traps quiescent cancer stem-like cells into S/G2/M-phases. Clin Cancer Res. December 1, 2013 19:6495-6505.
Fucci Induced Spheroid of HT29 Cell Line
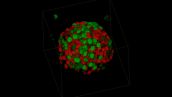
Image data courtesy of:
Dr. Yuji Mishima, Dr. Kiyohiko Hatake
Clinical Chemotherapy, Cancer Chemotherapy Center, Japanese Foundation for Cancer Research
Behaviors of Migration and Cell-cell Connection of Fibroblasts in A Multi-layered Myoblast Sheet
|
Image data courtesy of:
Eiji Nagamori, Ph.D Masahiro Kino-oka, Ph.D. Department of Biotechnology, Graduate School of Engineering, Osaka University |
Visualizing Retinoic Acid Signaling in A Zebrafish Embryo Using YFP as a Reporter

Image data courtesy of:
Satoshi Shimozono, Ph.D. Atsushi Miyawaki, M.D., Ph.D.
Laboratory for Cell Function Dynamics, Advanced Technology Development Core, RIKEN Brain Science Institute
Reference:
Shimozono S. et al. Visualization of an endogenous retinoic acid gradient across embryonic development. Nature 496, 363-366 (18 April 2013)
Observation of Antibody Dependent Cellular Cytotoxicity (ADCC)
|
RPMI 4788 cells (human colon cancer cell line) were treated with an antibody drug, cetuximab, and co-cultured with natural killer (NK) cells ADCC was observed using the FV10i after addition of NK cells
Cetuximab: Alexa Fluor 647 (red) NK cells: ZsGreen (green) Detection of dead cells: DAPI (blue) |
Image data courtesy of:
Dr. Yuji Mishima, Dr. Kiyohiko Hatake
Clinical Chemotherapy Department, The Cancer Chemotherapy Center of the Japanese Foundation for Cancer Research
Observation of the Effect of Anticancer Cisplatin with Different Concentrations
| Cell-cycle of HT-29 cells treated anticancer drug ,Cisplatin, was observed using time-lapse imaging of HT-29 expressing Fucci (a fluorescent cell-cycle indicator). The cells was treated by Cisplatin with different concentrations, control( 0 ug/ml), low(0.25 ug/ml), high(2.5 ug/ml) in 35 mm glass bottom dish after culturing for 48 hour. |
Image data courtesy of:
Dr. Yuji Mishima, Dr. Kiyohiko Hatake
Clinical Chemotherapy Department, The Cancer Chemotherapy Center of the Japanese Foundation for Cancer Research
Localization of Phosphoinositides During Cell–cell Fusion with Long Projections
| RAW264.7 cells expressing the reporter constructs in the presence of 1 µg/ml doxycycline were stimulated with 10 ng/ml RANKL for 48 h. Localization of PtdIns(4,5)P2 was visualized in red, while that of PtdIns(3,4,5)P3 was visualized in green. Frames were taken every 2 min for 34 min. Bar, 25 µm. |
Image data courtesy of:
Tsukasa Oikawa, Ph.D.
Department of Molecular Biology, Hokkaido University Graduate School of Medicine
Reference:
Oikawa T, et al. Tks5-dependent formation of circumferential podosomes/invadopodia mediates cell-cell fusion. J Cell Biol. 197(4):553-568(2012).
HeLa Cell*1
|
Dye: YFP (Actin)
Lens: 60XW NA1.2 Laser wavelengths: 473nm Time Interval: Every 3.5 minutes (TOTAL 12h) |
Mouse Brain
| Mouse brain fluorescently labeled with DAPI (nuclei - blue), Alexa 488 (Actin - green), Alexa 568 (Neurofilament - red). Image is comprised of 9 different regions of interest automatically acquired over 14 Z sections at 1024x1024 and stitched together using FV10i software. |
HeLa Cell*1
|
Time Interval: Every 20 minutes during a night
Green: GFP Magenta: Mito Tracker Red |
*1 Although it became one of the most important cell lines in medical research, it’s imperative that we recognize Henrietta Lacks’ contribution to science happened without her consent. This injustice, while leading to key discoveries in immunology, infectious disease, and cancer, also raised important conversations about privacy, ethics, and consent in medicine.
To learn more about the life of Henrietta Lacks and her contribution to modern medicine, click here.
http://henriettalacksfoundation.org/