Application of silicone immersion objectives to long-term 3D live-cell imaging of mouse embryo during development
Long-term 3D live-cell imaging of a mouse embryo during development
Advances in microscopy have revealed numerous phenomena that occur during embryonic development, and this has become a major research focus in the field of developmental biology. In particular, as confocal microscopes have come into more general use, this has enabled researchers to obtain sharp three-dimensional fluorescence images of proteins, DNA, and other molecules in the zygote and of individual cells during embryonic development.
With research in this area making rapid progress, confocal microscopy is being used for prolonged 3D live-cell imaging to capture dynamic change over time as well as for three-dimensional fluorescence imaging. Olympus developed silicone immersion objectives that enable high-contrast[PG1] , long-term 3D live-cell imaging.
This application note introduces an example of high-contrast 3D live-cell imaging during the in vitro development of a mouse embryo from the zygote to blastocyst stage over approximately 4 days using a silicone immersion objective.
This application note is based on a study conducted by Dr. Kazuo Yamagata, Associate Professor in the Department of Genetic Engineering, Faculty of Biology-Oriented Science and Technology at Kinki University and his collaborators that was published in Stem Cell Reports in June 2014.
The use of silicone immersion objectives for long-term 3D live-cell imaging of a MethylRO mouse early embryo during preimplantation development
1)Creating MethylRO mice to visualize epigenetic changes in living cells
Researchers generated a fluorescent probe by fusing a red fluorescent protein to the methyl-CpG binding domain (MBD) of methyl-CpG binding domain protein 1 (MBD1), which recognizes methylated DNA. They then inserted the gene by targeting the ROSA26 locus, which is known for its ubiquitous gene expression, and generated the MethylRO mouse, a genetically modified mouse strain expressing this probe throughout the entire body.

Figure 1. Neonate MethylRO mouse for visualization of methylated DNA (yellow arrows).
When irradiated with the excitation light, the entire body glows red through a filter (right panel).
2)Long-term 3D live-cell imaging of an early embryo during preimplantation development using a 60X silicone immersion objective
Dr. Yamagata and his collaborators used a confocal microscope for time-lapse 3D live-cell imaging of cells from a MethylRO mouse (Fig. 1) during early development of the preimplantation embryo over approximately 4 days.
The researchers used an Olympus silicone immersion objective designed for live cell observation. Silicone oil has a refractive index (ne≈1.40) close to that of living tissue (ne≈1.38). Spherical aberration, which occurs with oil or water immersion objectives due to a refractive-index mismatch with biological samples, is reduced in silicone immersion objectives, allowing researchers to achieve deep, high-contrast fluorescence imaging at a greater depth. In addition, silicone oil does
not dry out or become solid, compared to water or oil immersion, when used in a warm (37 °C) environment over 4 days, thereby supporting long-term, stable high-resolution 3D live-cell imaging.
Dr. Yamagata’s team previously used an oil lens and water immersion objective, the former standard for deep observation in live-cell imaging. By switching to a silicone immersion objective, the researchers were able to view fluorescently labeled methylated DNA (mCherry-MBD-NLS) within the nuclei from the surface to the inner region over approximately 4 days from the one-cell zygote to blastocyst stage.
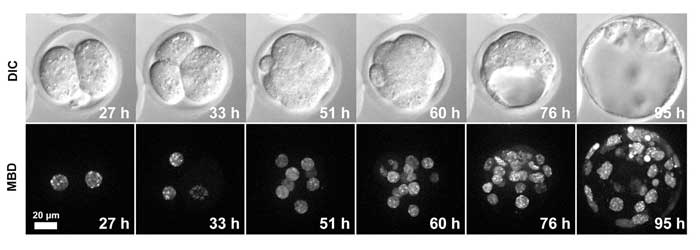
Figure 2. Live-cell imaging of a MethylRO embryo during pre-implantation development.
Changes in methylated DNA (mCherry-MBD-NLS) within the nuclei were observed over approximately 4 days.
Movie1. Time-lapse imaging over 82 hours of changes in methylated DNA in the nuclei (red: mCherry-MBD-NLS) and cells (green: CAG-EGFP).
| |
Movie2. Three-dimensional image of methylated DNA (mCherry-MBD-NLS) within nuclei of a blastocyst |
Figure 2 and Movie 1 above show that fluorescence photobleaching during long-term imaging was negligible, enabling clear imaging of methylated DNA within the nuclei. In Movie 2, a three-dimensional image of the entire 100 mm blastocyst was successfully obtained using an objective with both a high numerical aperture (1.3) and long working distance (0.3 mm)
Conclusion: The use of silicone immersion objectives is essential for the realization of deep, high-contrast 3D live-cell imaging over long periods of time.
Olympus’ line-up of 30/40/60/100X silicone immersion objectives offers both high numerical aperture (NA) and long working distance. Since the refractive index of silicone oil (ne≈1.40) is close to that of living tissue (ne≈1.38), spherical aberration induced by a refractive-index mismatch is reduced when observing thick tissues, thereby enabling high-resolution imaging. In addition, silicone oil does not dry out so there is no need to add more immersion liquid during an experiment. The silicone immersion objectives are compatible with the IX motorized inverted microscope series’ Z-Drift Compensation System IX-ZDC. With this system, researchers can obtain images that are constantly in focus and unaffected by temperature changes during long-term observation. In addition, the refractive index of silicone oil (ne≈1.40) and that of SCALEVIEW-A2 (ne≈1.38), an optical clearing agent supplied by Olympus, are comparable, minimizing refractive index mismatch when using this reagent. Silicone immersion objectives with high NA provide optimum performance when optically cleared specimens are used.
Imaging conditions
Imaging system: Research inverted microscope IX series
Objective: silicone immersion objective UPLSAPO60XS
Confocal scanner unit: CSU-X1 (Yokogawa Electric Corporation)
EMCCD camera: iXON3 DU897E-CS0 (Andor Technology)
This application note was prepared with the help of
Dr. Kazuo Yamagata, Associate Professor, Department of Genetic Engineering, Faculty of Biology-Oriented Science and Technology, Kinki University
For more details on the studies in this application note, please refer to the article below:
Ueda, Jun, Kazumitsu Maehara, Daisuke Mashiko, Takako Ichinose, Tatsuma Yao, Mayuko Hori, Yuko Sato, Hiroshi Kimura, Yasuyuki Ohkawa, and Kazuo Yamagata. "Heterochromatin dynamics during the differentiation process revealed by the DNA methylation reporter mouse, MethylRO." Stem cell reports 2, no. 6 (2014): 910-924.
Productos usados para esta aplicación
se ha añadido correctamente a sus marcadores
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.