In medical and life science research, both cell-level experiments and experiments involving animal models are very important. The number of studies that observe ecology under a microscope increases each year, a good portion requiring time-lapse intravital imaging of living specimens. A major issue for researchers doing such intravital imaging is shifting of the observation plane due to the pulsation of organs and the movement of the animal itself.
In addition to standard microscope systems, we work with customers to create customized solutions. Here, we present one example—our mouse adsorption fixing device, which we developed to help solve problems associated with intravital imaging in mice.
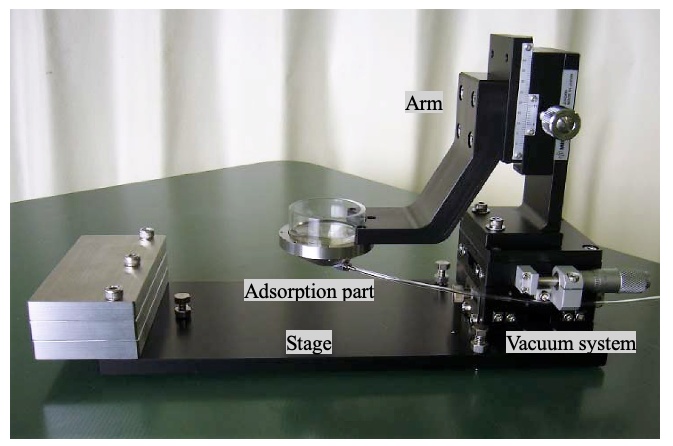
Example of a customized mouse adsorption fixing device
Problems for Researchers Conducting Long-Term Time-Lapse Intravital Imaging
In intravital imaging, the movement of the specimen and observation plane due to the pulsation of organs complicates time-lapse experiments. We conducted interviews with researchers who conduct long-term time-lapse experiments on living mice to gain a better understanding of their struggles. We learned:
- Compared with organs that can be taken out of the body such as the auricle and intestines, it’s difficult to observe organs such as the spleen and liver.
- Organs close to the heart are particularly susceptible to pulsation.
- It’s not only millimeter-order movements, such as pulsation, but also fine micrometer-order movements that make observation difficult.
EVIDENT’s Customized Solution for Intravital Imaging Issues
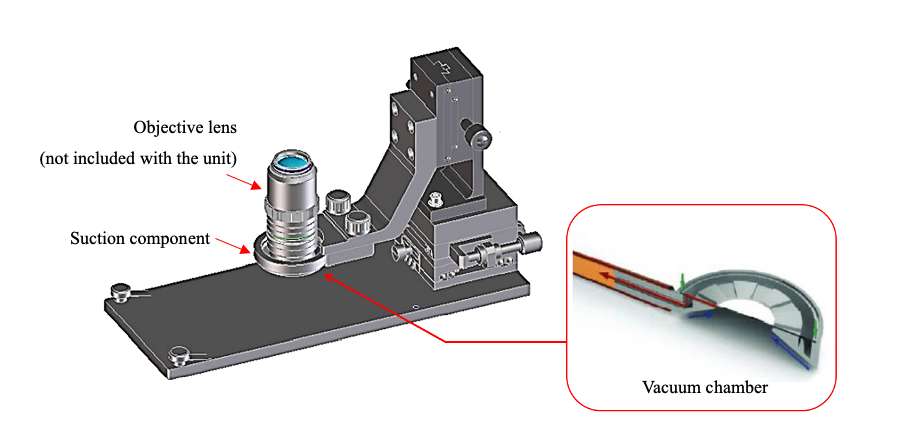
To suppress the pulsation of the mouse on the observation surface, we devised a method that uses suction to physically inhibit the movement of the animal in the millimeter order. Our device features a donut-shaped metal fitting and a suction pump to scoop up and fix the observation site with negative pressure. By minimizing the specimen’s movements, we succeeded in suppressing the influence of the large pulsation from the heart.
Solving the Secondary Problem of Retaining Sufficient Immersion Liquid
Immersion objective lenses are typically used for time-lapse observation of living organisms. However, only a small amount of immersion liquid can be retained on the observation surface because of surface tension. In addition, when trying to replenish the immersion liquid during the experiment, problems such as temperature changes and physical pressure cause the observation surface and observation part to become misaligned. To solve these issues, we also provided a mechanism in the mouse adsorption fixing device that can hold enough immersion liquid for long-term observation. This mechanism enables researchers to perform long-term observation without needing to replenish the immersion liquid.
Without our mouse adsorption fixing device, researchers were forced to fix the living body through trial and error for each experiment. With this device, it’s now possible to perform stable long-term time-lapse observation while maintaining biological activity.
See the Solution in Action: Watch These Examples of Time-Lapse Intravital Imaging
See for yourself how well the mouse adsorption fixing device works—watch these time-lapse videos captured in collaboration with Matsuda Laboratory of Kyoto University.
Related Videos
Observation of lymphatic cell movement due to laser ablation damage in lymph nodes by multiphoton microscopy
This time-lapse experiment involved the observation of ERK activity in liver cells using FRET (CFP-YFP). High ERK activity is shown in red, and low ERK activity is shown in blue. 30 minutes after the start of image acquisition, Kupffer cells, which are damaged by the laser and are lymphoid cells, gather at the inflammatory site.
- Image acquisition conditions
- Sample: Eisuke mouse
- Objective: XLPLN25XWMP
- Laser: 840 nm
- Zoom: 1x (left), 3x (right)
- Z position: 0 µm(bottom), 20 µm(upper)
- Interval: 40 sec.
- Total time of experiment: Approximately 2.5 hours
This video recorded the time-lapse observation of ERK activity in lymph nodes by FRET (CFP-YFP). High ERK activity is shown in red, and low ERK activity is shown in blue. 30 minutes after the start of image acquisition, laser damage occurred, and immediately after that, lymphoid cells around the damaged area stopped moving.
Related Videos
Observation of lymphatic cell movement due to laser ablation damage in lymph nodes by multiphoton microscopy
This video recorded the time-lapse observation of ERK activity in lymph nodes by FRET (CFP-YFP). High ERK activity is shown in red, and low ERK activity is shown in blue. 30 minutes after the start of image acquisition, laser damage occurred, and immediately after that, lymphoid cells around the damaged area stopped moving.
- Image acquisition conditions
- Sample: Eisuke mouse
- Objective: XLPLN25XWMP
- Laser: 840 nm
- Interval: 30 sec.
- Total time of experiment: Approximately 1 hours
Related Videos
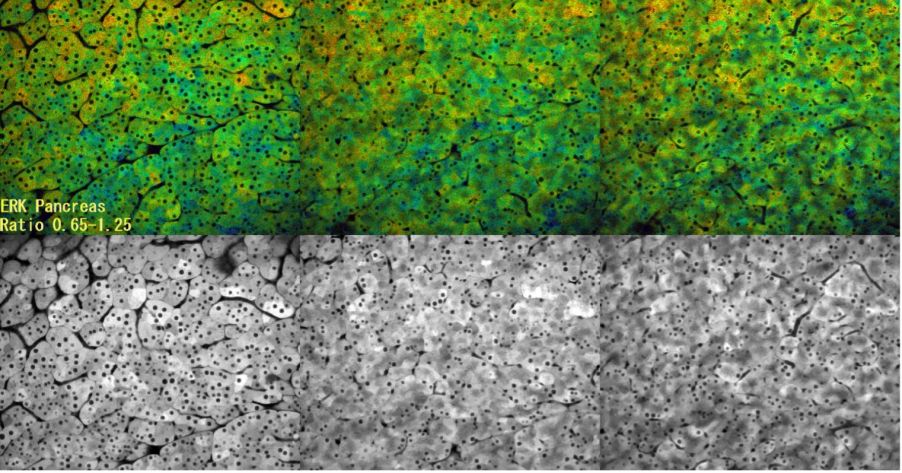
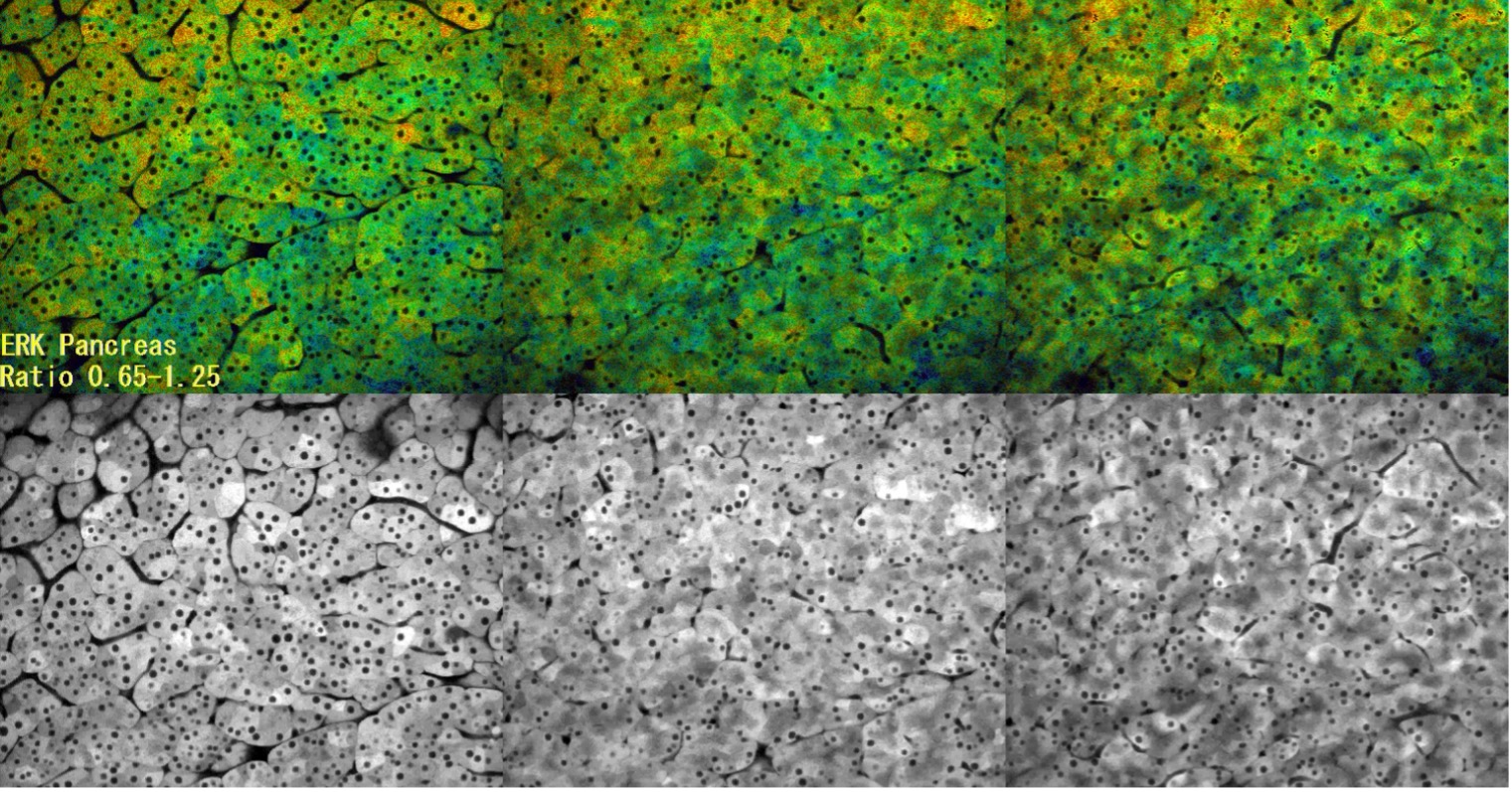
Observation of ERK activity in pancreatic cells by multiphoton microscopy
In this time-lapse capture, ERK activity was observed using FRET (CFP-YFP). High ERK activity is shown in red, and low ERK activity is shown in blue. This was a control experiment to validate that the imaging conditions do not alter ERK activity. Since there was no change even after 3 hours of observation, we can be conclude that there is no effect on ERK activity even if observed while aspirating with a fixture.
- Image acquisition conditions
- Sample: Eisuke mouse
- Objective: XLPLN25XWMP
- Laser: 840 nm
- Z position: 0 µm (left), 7.5 µm (middle), 15 µm (right)
- Interval: 2 min.
- Total time of experiment: Approximately 3 hours
Interested in This or Other Customized Microscopy Solutions?
In addition to this mouse adsorption fixing device, Evident offers other extended solutions for standard systems with advanced microscopy technology. However, these customized solutions are not available in some countries or regions. Learn more at our Customized Solutions page.
*The mouse adsorption fixing device is a product that uses our patented technology (Patent No. 6037732).
**These microscope images and videos in this blog post were made using mice expressing FRET probes with the cooperation of the Matsuda Laboratory of Kyoto University.