Each year, Nature Methods reviews the technologies and approaches that have advanced the life science industry over the past year, then selects the most popular and influential ones. In 2017, Nature Methods named organoids the Method of the Year. Since then, the research on organoids has been fruitful thanks to the tireless efforts of many researchers.
Simply put, organoids are three-dimensional (3D) tissue cultures that may be derived from tissue or stem cells. Organoids are used for a wide range of research since they are essentially simplified and miniaturized versions of human tissues or organs. They closely simulate the physiological structure and function of in situ tissues and can stably maintain genetic information through numerous passages.
Due to their ability to mimic parts of the human body, researchers can use organoids to:
- Gain insight into the developmental process of organs
- Test the function of drugs
- Develop regenerative therapies
This post will explore some of the latest organoid research and share the ways microscope imaging systems are advancing this important work.
Advances in Organoid Research
Researchers continue to produce new research using organoids. Here are some notable studies:
1. Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-tumoral Heterogeneity
In 2019, researchers from the University of Pennsylvania published a paper entitled "A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-tumoral Heterogeneity” in Cell. It reported the establishment of a patient-derived glioblastoma organoid (GBO) model and biobank.
The study showed that the GBO retains the key characteristics of glioblastoma, and it can be immediately used to develop treatment strategies for patients. The biobank established by the researchers provides rich resources for basic and translational studies of glioblastoma.
The researchers documented the growth process and morphological changes of the organoid observed under a microscope. They also observed the expression patterns of some protein markers in the organoid using confocal laser scanning microscopy.
This work demonstrated the apparent heterogeneity in the morphology and characteristics of organoid cells, as well as their highly similar cellular composition to the parental tumor.
2. Chromatin Accessibility Dynamics in a Model of Human Forebrain Development
In 2020, the paper entitled "Chromatin accessibility dynamics in a model of human forebrain development” was published in Science. Here, researchers described the culture process to create and the intended use of the human forebrain organoid. They demonstrated how human forebrain organoids can self-assemble into different parts of the forebrain.
More excitingly, the researchers also found a way to extend the lifespan of human forebrain organoids up to 300 days. That’s enough time to observe the development of forebrain organoids into more complex structures.
3. Amplification of Human Interneuron Progenitors Promotes Brain Tumors and Neurological Defects
In 2022, the paper “Amplification of human interneuron progenitors promotes brain tumors and neurological defects" was published in Science. This study revealed the specific human developmental processes responsible for malformations of cortical development (MCDs), which may lead to developmental delays and epilepsy in children.
The researchers established a human brain organoid model for tuberous sclerosis complex (TSC) and identified a specific neural stem cell type—the caudal late interneuron progenitor (CLIP). In TSCs, CLIP cells overproliferate, producing excess interneurons, brain tumors, and cortical malformations.
Organoids are promising models for studying development, diseases, and drugs. They also have broad prospects in other fields, such as regenerative medicine. Yet, there are still many challenges in culture, observation, and quantitative data analysis of organoids as their culture conditions and structures are more complex than those of attached cells.
3 Ways Microscope Imaging Advances Organoid Research
Fortunately, microscope imaging equipment can help with these challenges. Here are three ways microscope imaging systems and software are advancing organoid research:
1. Quality Control in the Culture of Organoids
The typical preparation method of organoids is to isolate embryonic cells or induced pluripotent stem cells, then culture these cells on support media (such as Matrigel) to enable their three-dimensional growth.
Cytokines, growth factors, and small molecules are added to the medium to activate or inhibit specific signaling pathways involved in forming organoids. The signaling pathways are the same as those mediating the development and maintenance of homeostasis of the equivalent organs in vivo.
According to research by Dr. Takanori Takebe from the Tokyo Medical and Dental University Institute of Research, the efficiency of subsequent differentiation into organoids is greatly affected by differences in the early stage of proliferation during the maintenance culture. He found that in the iPS cell lines capable of differentiation, colonies formed from most of the single cells adhered after passage in the early stage of proliferation.
Dr. Takebe hypothesized that the higher frequency of cell death in the early stages of maintenance culture in differentiation-resistant iPS cell lines causes a lower number of iPS cell colonies, which ultimately decreases their differentiation efficiency into liver bud organoids. As a result, the reproducible formation of organoids requires both the development of more optimal protocols for the differentiation of organoids and the optimization of culture for the original undifferentiated iPS cells. To gain new insights and improve experimental protocols, Dr. Takebe uses our incubation monitoring system.
Using the CM30 system for the acquisition and analysis of time-lapse data of cultured samples, researchers can understand the status of iPS cells during cell culture and identify key factors for increasing differentiation efficiency to further improve experimental protocols.
Because of the long culture time of organoids and the high cost of media and additives needed to maintain their growth, researchers must pay special attention to the culture process of organoids and their growth, as well as avoid contamination.
Thanks to the remote monitoring of the CM30 system, there’s no need to enter the clean room to remove the specimens from the incubator for microscope examination. This greatly increases the experimental efficiency and reduces the contamination risk.
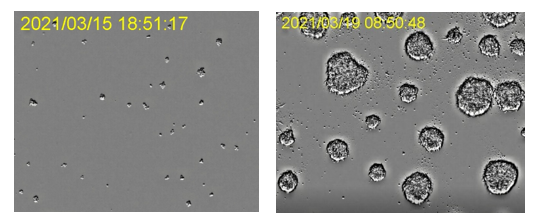
Development of organoids in an incubator. Images captured using the CM incubation monitoring system. Image data courtesy of ACEL, Inc.
2. Microscope Imaging of Organoids
Since organoids are 3D cell cultures with a certain volume, microscope imaging devices that can acquire multi-layer Z-axis image information for 3D imaging are preferred. This capability enables you to obtain complete information on the morphological characteristics and internal cellular structures of organoids in imaging.
Laser Scanning Confocal Microscopy for Organoid Imaging
Laser scanning confocal microscopes are suitable for acquiring 3D volumetric images of an organoid. Thanks to the pinhole in front of the detector that blocks out-of-focus background, only the information at depth-of-interest is captured with high axial resolution.
Our FLUOVIEW™ FV4000 confocal laser scanning microscope features precision imaging with our SilVIR™ detector, which enables a high signal-to-noise ratio, more accurate imaging, and high spatial and spectral resolution. The SilVIR detector uses our patented* technology to enable high dynamic range photon counting for precise 3D imaging data. The technology also makes it possible to reconstruct precise 3D images, which is ideal for investigating organoid structures.
*Patent number US11237047.
The FV4000 system also provides a proven near-infrared (NIR) solution. NIR imaging provides deep penetration, low phototoxicity, and low tissue autofluorescence interference. As a result, it works with various fluorescent contrasts in the visible range for crosstalk-free multicolor fluorescence imaging of organoids with the potential for long-term monitoring of organoid activities.
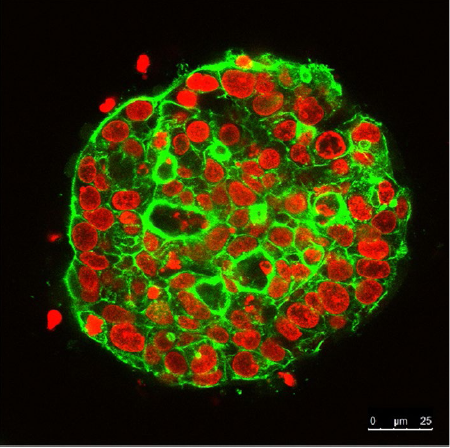
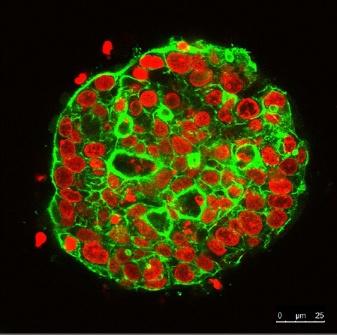
Continuous observation of 3D cultured cell spheres for 21 days using the FLUOVIEW confocal laser scanning microscope. The nucleus is labeled with SYTOX Orange (red). The cytoskeleton is labeled with Alexa Fluor 488 (green).
Multiphoton Microscopy for Organoid Imaging
To obtain a complete image of an organoid with a large volume, there’s a stricter demand for imaging depth. Multiphoton microscopes are suitable microscope imaging systems for deep imaging of organoids.
Here’s an overview of how these systems can help with deep imaging of organoids:
Our FV4000MPE multiphoton microscope has an advanced optical design to optimize the sensitivity and resolution in deep imaging. A wavelength range of 400–1600 nm supports more efficient IR excitation without compromising visible wavelength detection.
Its detection optical path with a large clear aperture and high efficiency supports pooling of more emission signals, especially scattered photons at large incident angles. In addition, the SilVIR detector at the core of the system offers exceptionally low noise and high sensitivity with a high signal-to-noise ratio across the visible to near-infrared wavelength range.
These optical capabilities enable you to capture information deeper inside an organoid. TruResolution™ objectives improve the brightness and resolution of deep imaging with automatic spherical aberration compensation. These high-performing optics enable more detailed information in a 3D image to be captured at all levels.
Super-Resolution Microscopy for Organoid Imaging
There are even greater demands on the imaging system for organoids with a large volume. The imaging system must acquire Z-stack images and splice the acquired images, which requires high-speed image acquisition. This is where spinning-disk confocal microscopy comes in.
While laser scanning microscopes use a single pinhole, spinning-disk confocal microscopes use an opaque disk with hundreds of pinholes that rotate at high speeds. The entire sample is imaged at once rather than point by point, significantly increasing imaging speed and reducing photodamage.
Our IXplore™ SpinSR super-resolution microscope is a spinning-disk confocal system that features fast imaging, high sensitivity, low phototoxicity, and scalable super-resolution modules with resolutions as fine as 120 nm. These capabilities support quick Z-stack imaging and image splicing of organoids.

IXplore SpinSR super-resolution microscope system.
The IXplore SpinSR system also works with our series of silicone oil objectives. These optics enable sharp super-resolution images with less blurring even in deep imaging of organoids.
Our silicone oil objectives use a special silicone oil with a refractive index of 1.40 as the immersion medium. This satisfies the requirements for acquiring high-resolution images (higher than water immersion objectives) and observing highly scattering specimens, such as organoids and other thick specimens.
3. Quantitative Analysis of Organoid Images
So far, we’ve discussed how you can acquire clear macro and micro images of organoids using high-end microscope imaging equipment, such as laser scanning confocal, multiphoton, and super-resolution microscopes. These images let researchers observe the fine structure of each cell or even the subcellular level inside a 3D specimen.
For life science research, it is not enough to only observe the details of a specimen. In experiments using organoid models for efficacy validation and toxicological analysis of drugs, qualitative and quantitative analyses of the morphology of the organoid and its internal cells are also required. For example, comparing the differences among multiple organoids at different drug dosage concentrations in a multi-well plate can lead to more convincing statistical data.
To meet these application needs, we developed the NoviSight™ cell analysis software for complex 3D cell identification and analysis of specimens, such as cell spheroids or organoids cultured in multiwell plates.
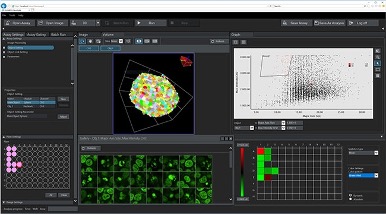
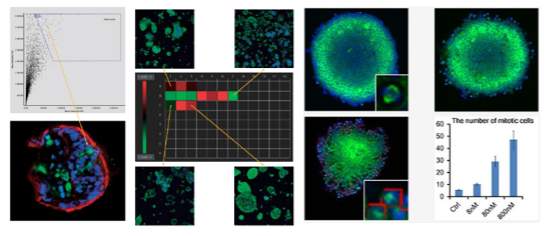
NoviSight software’s user interface. This example shows a quantitative analysis of mitotic cells inside cell spheroids treated with different paclitaxel concentrations.
Here are some of its helpful features for organoid research:
- Unique True 3D cell analysis technology can help to realistically reproduce the spatial morphology of your samples
- Multiparametric measurement module can help you quickly identify the organoid and cell components and obtain useful data, such as volume, surface area, spatial distance, and fluorescence intensity
- Interactive user interface lets you easily match cell images to their statistics for accurate statistical analysis of data
Because organoids can be used to closely simulate the corresponding human tissues at both the genetic and morphologic level, they have broad application prospects in the simulation of the development process, disease research, clinical immunity, drug sensitivity of tumors, regenerative medicine, and precision medicine.
Despite this, it is important to remember that organoids are still an emerging technology. Limitations exist in the culture, quality control, and experimental reproducibility of organoids. There’s still a long way to go for basic research, application, and translation of organoids.
With a rich history in optics and microscopy, Evident is committed to supporting organoid researchers throughout their experiment process. By providing complete solutions for organoid research, from sample preparation to 3D data acquisition and analysis, we contribute toward our mission of making the world a healthier and safer place.
Related Content
E-Book: Imaging and Analysis of Advanced 3D Cellular Models
Drug Viability Testing of 3D Cancer Spheroids Using Automated Macro-to-Micro Imaging