Fluorescent Image Analysis–3D Spheroid Invasion in a Collagen Gel
Confocal images of cancer spheroid invasion in a collagen gel were quantitatively evaluated using NoviSight™ software to gage the impact of an anti-cancer drug that targeted the factor related to tumor invasion.
Objectives
The metastatic cascade can be broadly separated into three main processes: invasion, intravasation, and extravasation. The first step, tumor invasion, is fundamental in tumor progression and is the driving force for metastasis. Tumor invasion is also composed of three processes: degradation of the extracellular matrix (ECM), intercellular adhesion detachment, and cell migration. ECM is a collection of extracellular molecules, such as collagen, fibronectin, laminin, and proteoglycan, and forms an intricate network. Tumor cells collectively migrate while repeating degradation and reconstruction of ECM. To evaluate invasion capacity or drug efficacy, a wound-healing assay or transwell assay is commonly used. These traditional assays are simple and inexpensive, but they lack the tumor's microenvironment and the cells lose relevant properties, such as polarization and cell to cell communication. Therefore, these artificial assays contribute to the poor predictive value of compound efficacies between in vitro and in vivo experiments. To recreate the tumor microenvironment, a three-dimensional in-gel invasion assay has been gradually expanded. This assay can recreate the tumor microenvironment by embedding tumor spheroids in a gel matrix. The embedded spheroids gradually sprout into the gel. This assay can closely mimic tumor morphology or the collectiveness of invasion. In this study, NoviSight™ software and the 3D in-gel invasion assay were used to quantitatively analyze the effect of anti cancer drugs on invasive spheroids. The study demonstrates the software's ability to quantify the invasion of complex shaped tumors.
Preparation of samples
A cell suspension of human fibrosarcoma cells HT1080 were seeded in a U-bottom 96-well plate (Corning® Inc.) (Day 1). On Day 3, HT1080 spheroids were embedded in matrigel (Corning® Inc.), and a culture medium containing Batimastat, an anticancer drug that specifically inhibits matrix metalloproteinase (MMP), was added over the matrigel. On Day 5, cell nuclei were stained with Hoechst33342.
Conclusion
Acquisition and analysis of fluorescent images
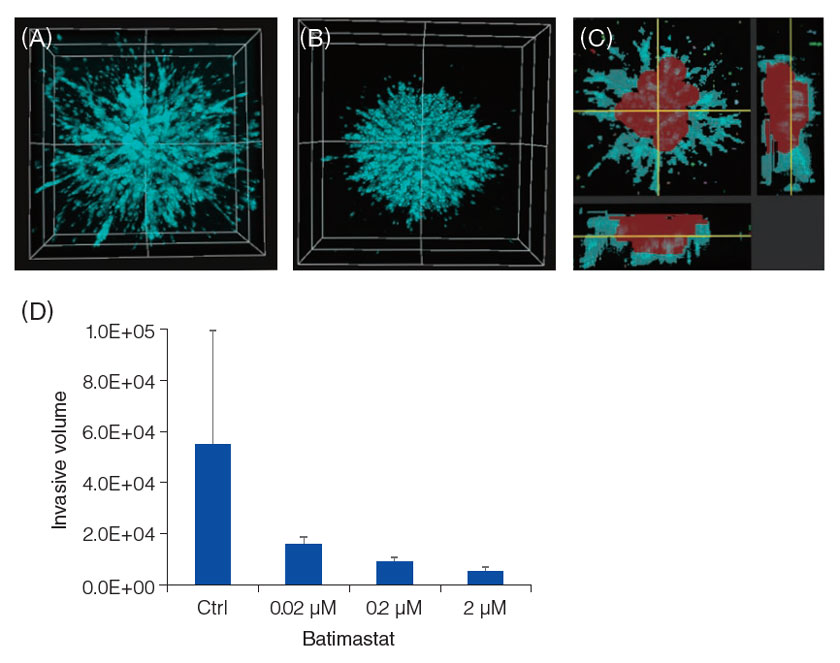
Observations were made using the FV3000 laser confocal microscope. On Day 5, a non-treated spheroid showed powerful invasion (A), however, invasion was inhibited in the presence of Batimastat (B). The invasion area and its complex 3D structure was recognized by NoviSight™ software (C: the red area is the center of a spheroid, and the blue area is the projection portion of invasion). Using the software's volume analysis function, we were able to determine that Batimastat inhibited HT1080 spheroid invasion in a dose dependent manner (D).
U-bottom 96-well plate is a registered trademark of Corning®Inc.
Olympus is a registered trademark, and NoviSight and Insightful Analysis, Intelligent Answers are trademarks of Olympus Corporation.
Products Related to This Application
was successfully added to your bookmarks
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.