Cell Quality Evaluation with the CM20 Incubation Monitoring System : Improve the Reproducibility of Experiments through the Characterization of Cells
Introduction
In research using cultured cells, achieving experiment reproducibility is often a challenge. To overcome this problem, “stronger experimental design” and “better statistics” are proposed as countermeasures.*1 However, acquiring the quantitative data required to deploy this measure places a burden on researchers. In this application note, we introduce an approach to improve the reproducibility of experiments by acquiring quantitative data in cultures using the CM20 system, alleviating the onus of data gathering placed on researchers.
Conventional Issues: Difficulty in Obtaining Quantitative Data at the Culture Preparation Stage
Generally, the quality judgment and the operation timing of the culturing cells, which is the preliminary stage of the experiment, are entrusted to the subjective judgment of the operator, contributing to considerable variation in results among the operators. Furthermore, when collecting quantitative data of cells, it is necessary to pick out a part of the sample, and there is a lack of practical tools to measure a large amount of data nondestructively.
Our Solution: Acquisition of Quantitative Data Using the CM20 Incubation Monitoring System
Since the CM20 incubation monitoring system can automatically observe the cell sample at stable intervals without removing it from the vessel, quantitative data can be acquired without the intervention of the researcher or the risk of destabilizing the cell culture environment. It is also possible to automatically measure the number of cells from the acquired image and calculate the doubling time, which is an index of cell proliferation ability.
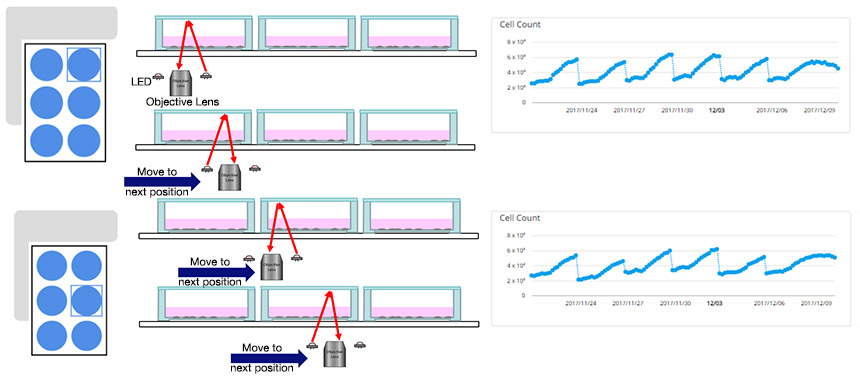
Fig.1 CM20 can automatically measure quantitative data during passage of cells seeded in six-well plate
Experiment to Verify Improved Reproducibility: Relationship between Cell Characteristics during Passage Using HUVECs and Differentiation Induction
The following is an example of the successful use of the quantitative data of cell passage as a quality.
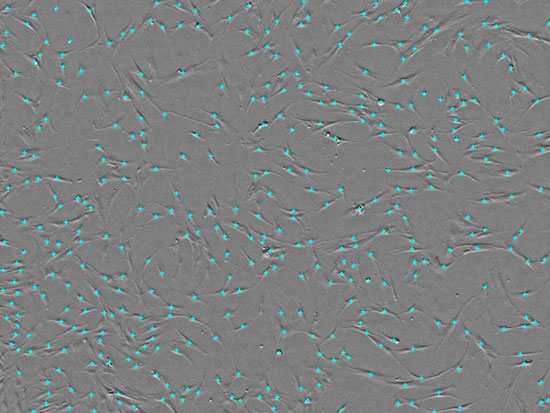
Fig.2 Acquiring MSC images and counting cells with CM20
Experiment to Verify Improved Reproducibility: Relationship between Cell Characteristics during Passage Using HUVECs and Differentiation Induction
The following is an example of the successful use of the quantitative data of cell passage as a quality evaluation. Cell morphology and doubling time were analyzed from a large number of images acquired during the subculture of human umbilical vein endothelial cells (HUVECs). Our findings indicated that there is a high correlation of the HUVECs’ doubling time during subculture with their angiogenic potential. In this case, it was demonstrated that experiments with high reproducibility can be designed by using up to Passage 8 (P8), which is where changes in proliferative ability occur.
(This result was obtained using the prototype of the CM20 system. Refer to the article in Scientific Reports for details of the experiment*2)
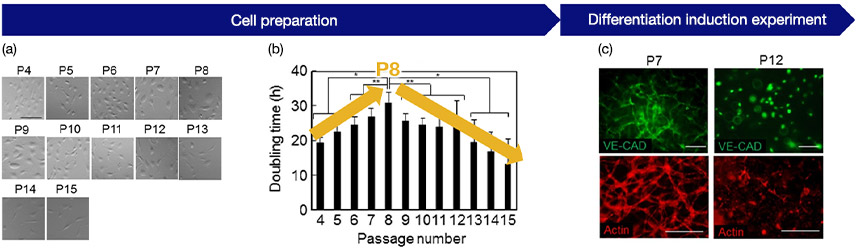
Fig.3 (a) Cell morphology by passage number: Recording image data at each passage, (b) Relationship between passage number and growth rate: It shows that the proliferation rate changes at P8, (c) Angiogenic potential and passage number of HUVEC: Shows successful angiogenesis at P7 and poor angiogenesis at P12
Testimonial
| In the field of life sciences, it is regarded as a problem that experiments conducted in the past cannot be reproduced. With the conventional method, slight changes during subculture had to be visually perceived by experts in the laboratory, but by using a newly developed monitoring system, a large amount of images were acquired and the data was analyzed to determine the quality of cells. In the future, we hope that in fields of life sciences such as regenerative medicine, we will improve the reproducibility of experiments by acquiring data during culture and evaluating the quality. |
|
Prof. Junji Fukuda
Division of Materials Science and Engineering, Faculty of Engineering Chemical Engineering and Life Science, Yokohama National University | |
References
*1) Monya Baker, "1,500 scientists lift the lid on reproducibility", nature 533, 452–454 (26 May 2016)
*2) Tatsuya Osaki, Tatsuto Kageyama, Yuka Shimazu, Dina Mysnikova, Shintaro Takahashi, Shinichi Takimoto & Junji Fukuda, "Flatbed epi relief-contrast cellular monitoring system for stable cell culture", nature Scientific Reports ISSN 2045-2322 (online) (15 May, 2017)
Products Related to This Application
was successfully added to your bookmarks
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.
