The Olympus Microscopy Resource Center galleries include images of fluorescent specimens, as well as darkfield, phase contrast, and Hoffman modulation contrast photomicrographs. In addition, the gallery features streaming video and images from featured microscopists.
Bovine Pulmonary Artery Endothelial Cells (BPAE Line)
The BPAE cell line was initiated in January 1979 by P. Del Vecchio from the main stem of a pulmonary artery belonging to a young cow (Bos taurus). Pulmonary arteries, which extend from the heart to the lungs, are the only arteries in the mammalian body that carry dark, unoxygenated blood. The BPAE line of endothelial cells is positive for bovine diarrhea virus, one of the most important known bovine viral pathogens, which causes a broad array of clinical syndromes that result in significant losses in the beef industry each year. BPAE cells are also positive for angiotensin converting enzyme (ACE), an enzyme that is intricately involved in the maintenance of blood pressure and volume. Due to this fact, BPAE cells are often utilized in hypertension research as well as studies of atherosclerosis and coronary heart disease.
Mouse Hemangioendothelioma Endothelial Cells (EOMA Line)
The EOMA cell line was derived in 1980 from a mixed hemangioendothelioma present in an adult mouse (Mus musculus). Hemangioendothelioma is the term utilized to describe a varied group of vascular tumors that usually appear as red or blue nodules and tend to behave biologically in a manner that can be classified as falling between a benign hemangioma and malignant angiosarcoma. EOMA cells are tumorigenic in syngeneic mice and exhibit characteristic endothelial cell properties. The cells synthesize a number of cellular products including angiotensin-converting enzyme (ACE), thrombospondin, Cathepsin L, endostatin, and interleukin-6. Surface receptors for acetylated low-density lipoprotein are expressed by EOMA cells, as is the vascular addressin, a tissue-specific endothelial cell adhesion molecule identified by antibody MECA-99.
Human Brain Glioma Cells (U-118 MG Line)
The U-118 MG cell line is one of several cell lines derived from malignant gliomas by J. Ponten and associates in the late 1960s. The source for the cells of this particular line was a 50-year-old Caucasian male. The morphology of U-118 MG line is mixed and both glioblastoma and astrocytoma cells are present. U-118 MG cells are very similar to the glioblastoma U-138 MG cell line, though the line is supposed to have been derived from a different source. Studies have found that both of these lines have identical VNTR (variable number of tandem repeats) patterns and similar STR (short tandem repeat) patterns in DNA analysis experiments. They also have in common at least six derivative marker chromosomes. Mycoplasma contamination was detected and eliminated from the U-118 MG cell line in 1987 through treatment of cultures with BM-cycline over a six-week period. The cells have been demonstrated to be tumorigenic in nude mice subcutaneously inoculated.
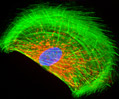
Rat Jejunum Myenteric Plexus Enteroglial Cells (EGC/PK060399egfr Line)
Enteroglial cells (known by the acronym: EGC) are believed to be an important part of the enteric nervous system, but much about the function of these cells is still unknown. To help facilitate future studies of the cells, A. Ruhl and coworkers developed a new method in 2001 for isolating and purifying enteroglial cells from the myenteric plexus, a network of nerve fibers located in the intestinal wall. The Ruhl method involved enzymatic dissociation of myenteric plexus samples, the purification of enteric glial cells via complement-mediated cytolysis of contaminating cells, and transformation by retroviral gene transfer. The group then characterized the resulting clones both immunohistochemically and by dot-blot analysis. As a result of their efforts, a number of transformed EGC lines that retain their glial and functional characteristics have been established.
 Rat Jejunum
Rat Jejunum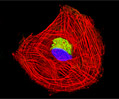 Rat Jejunum
Rat Jejunum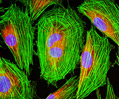 Rat Jejunum
Rat Jejunum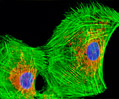 Rat Jejunum
Rat Jejunum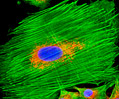 Rat Jejunum
Rat Jejunum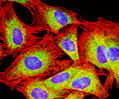 Rat Jejunum
Rat Jejunum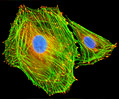 Rat Jejunum
Rat Jejunum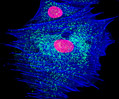 Rat Jejunum
Rat Jejunum Rat Jejunum
Rat Jejunum Rat Jejunum
Rat Jejunum Rat Jejunum
Rat Jejunum Rat Jejunum
Rat Jejunum
Rat Kidney Mesangial Cells (RMC Line)
The RMC cell line was derived from the kidney tissue of a 3-month-old male rat (Rattus norvegicus) belonging to the Sprague-Dawley strain. The mesangial cells were immortalized at passage eight with the plasmid pSV3-Neo, which codes simian virus 40 (SV40) large T-antigen, and are maintained in the presence of the antibiotic, G-418. The rat kidney cells express normal genes of the wild type mesangial cells and grow adherently to glass and polymer surfaces in monolayer culture. RMC cells are positive for desmin and vimentin, but are negative for cytokeratin 8. Mesangial cells are specialized cells usually associated with glomeruli that are crucial for kidney function. Thus, the RMC line and other lines of mesangial cells are widely utilized in kidney research.
Transformed Mouse Cerebellum Microglial Cells (C8-B4 Line)
Microglia are specialized macrophages that are very important for their role in protecting the central nervous system. C8-B4 is a spontaneously transformed microglial clone of a cell line originally derived from the cerebellum of an 8-day-old mouse (Mus musculus) in 1984. This initial organ culture was used to establish several distinct astroglial cell lines. The C8-B4 clone was created in 1996, and the neuronal cells grow adherently in culture. Classical microglial markers, including MAC1, F4/80, and 2-4G2, are expressed by the C8-B4 clone, which appears to be derived from a microglial precursor since it reportedly does not express differentiation antigens present during the early stage of the monocytic lineage. C8-B4 cells produce and release large amounts of glutamate, a substance that typically functions as a neurotransmitter.